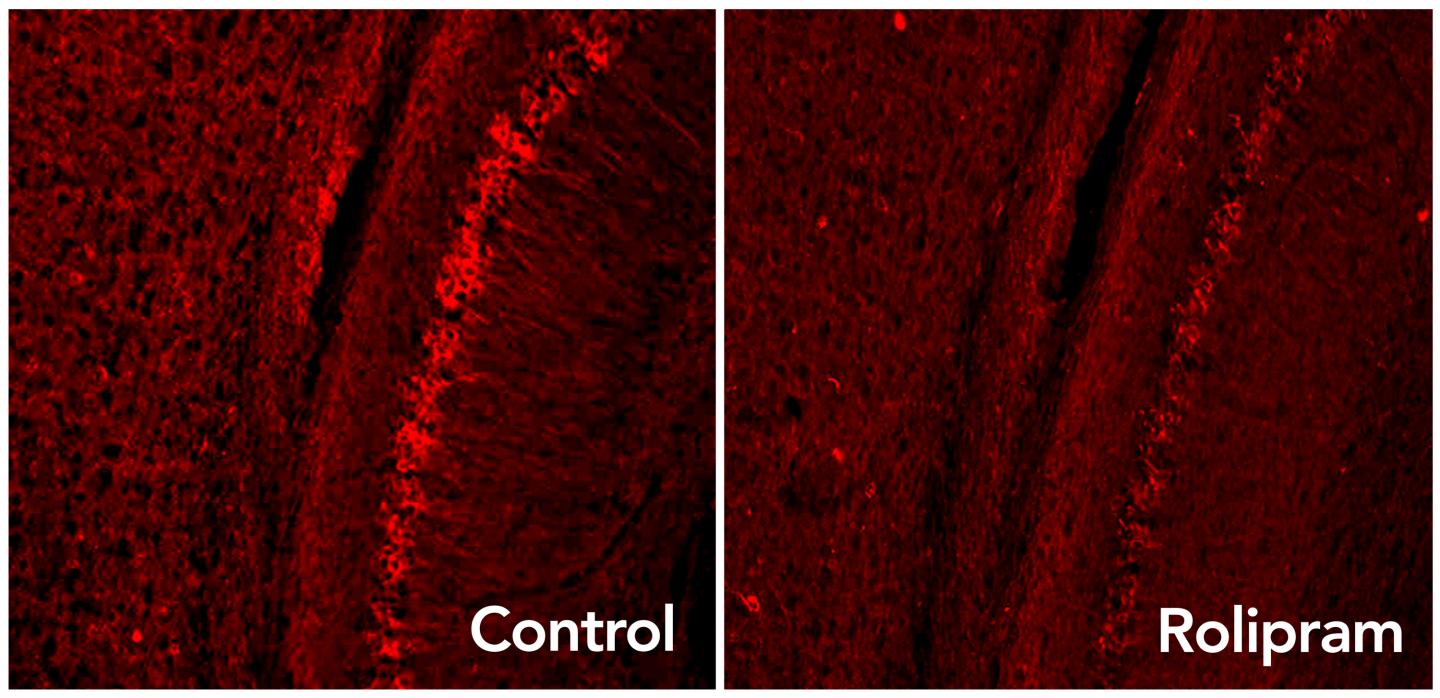
Rolipram drug activates the brain’s garbage disposal system, eliminating excess tau proteins (glowing red dots) associated with neurodegenerative diseases such as Alzheimer’s. (credit: Laboratory of Karen Duff/Columbia University Medical Center)
Rolipram, a drug that boosts activity in the brain’s “garbage disposal” system, can decrease levels of toxic proteins associated with Alzheimer’s disease and other neurodegenerative disorders and improve cognition in mice, a new study by neuroscientists has found.
Rolipram causes nausea, but similar drugs do not, and could be tested in clinical trials quickly, the researchers say.
“This has the potential to open up new avenues of treatment for Alzheimer’s and many other neurodegenerative diseases,” said study leader Karen E. Duff, PhD, professor of pathology and cell biology at Columbia University Medical Center (CUMC) and New York State Psychiatric Institute (NYSPI).
A “garbage-disposal” switch
To remain healthy, brain cells must continually clear out old, worn, or damaged proteins. This task is performed by a small molecule called the proteasome, which works like a kitchen garbage-disposal system, grinding up the old proteins so they can be recycled into new ones. However, in neurodegenerative diseases such as Alzheimer’s, Parkinson’s, and Huntington’s, proteins tagged for destruction accumulate in the brain’s neurons. This suggests that the cell’s proteasomes are impaired.
The cause for this: tau — a protein that accumulates in Alzheimer’s and other brain diseases — sticks to the proteasome and jams up the protein garbage-disposal process, the researchers first discovered (using a genetically engineered mouse).
In the new research, administering rolipram activated the proteasome and restored protein disposal. The drug also improved memory in diseased mice to levels seen in healthy mice.
Rolipram has been tested before in mice, and was shown to improve memory. But the new research shows a previously unknown function of the drug: it produces a physical change in the proteasome and increases its activity.
Should ‘clear out everything at once’
Duff says we still don’t know exactly which form of a particular protein is toxic to the brain, which has made it difficult to develop drugs to treat neurodegenerative diseases. “In Alzheimer’s disease, the problem is compounded because several types of abnormal protein can accumulate in a person’s brain, including amyloid, tau, alpha-synuclein, and TDP43.
However, the researchers think that “a well-functioning proteasome will be able to clear out everything at once,” she says — including Alzheimer’s, frontotemporal degeneration, Huntington’s, and Parkinson’s.
The study was published Tuesday (Dec. 22) in the online edition of Nature Medicine. The National Institute of Health’s National Institute of Neurological Disorders and Stroke provided funding for the study.
Abstract of Tau-driven 26S proteasome impairment and cognitive dysfunction can be prevented early in disease by activating cAMP-PKA signaling
The ubiquitin proteasome system (UPS) degrades misfolded proteins including those implicated in neurodegenerative diseases. We investigated the effects of tau accumulation on proteasome function in a mouse model of tauopathy and in a cross to a UPS reporter mouse (line Ub-G76V-GFP). Accumulation of insoluble tau was associated with a decrease in the peptidase activity of brain 26S proteasomes, higher levels of ubiquitinated proteins and undegraded Ub-G76V-GFP. 26S proteasomes from mice with tauopathy were physically associated with tau and were less active in hydrolyzing ubiquitinated proteins, small peptides and ATP. 26S proteasomes from normal mice incubated with recombinant oligomers or fibrils also showed lower hydrolyzing capacity in the same assays, implicating tau as a proteotoxin. Administration of an agent that activates cAMP–protein kinase A (PKA) signaling led to attenuation of proteasome dysfunction, probably through proteasome subunit phosphorylation. In vivo, this led to lower levels of aggregated tau and improvements in cognitive performance.
