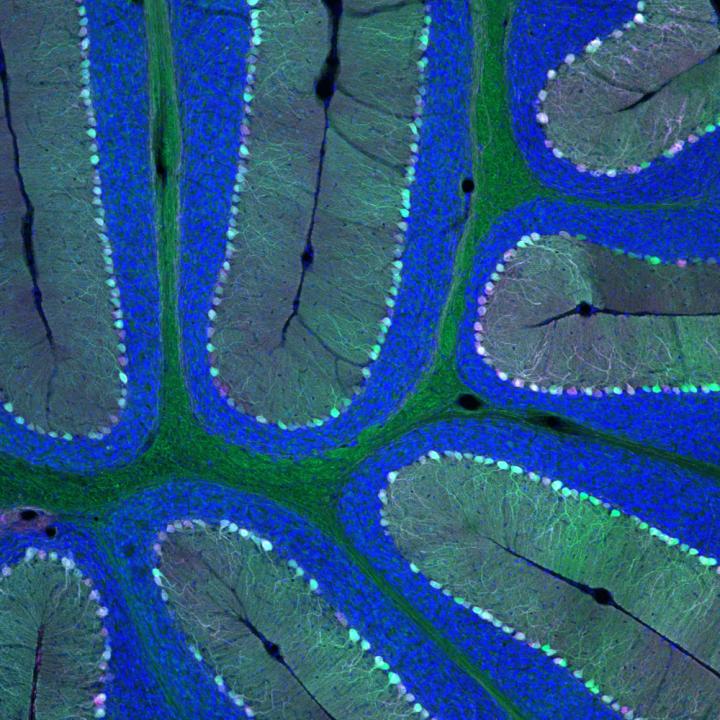
As a test of penetrating the blood-brain barrier using a harmless virus (AAV-PHP.B), green fluorescent protein (GFP) was created in the mouse cerebellum via delivery of the GFP gene via the virus. Purkinje neurons (shown in magenta) are responsible for sending signals out of the cerebellum. (credit: Ben Deverman and the Gradinaru laboratory/Caltech)
Caltech biologists have modified a harmless virus to allow it to enter the adult mouse brain through the bloodstream and deliver genes to cells of the nervous system.
The modified virus could lead to novel therapeutics to address diseases such as Alzheimer’s and Huntington’s, help researchers map the brain, and target cells in other organs, according to Ben Deverman, a senior research scientist at Caltech and lead author of a paper describing the work in the February 1 online publication of the journal Nature Biotechnology.
A viral penetration strategy
The blood-brain barrier (BBB) allows the body to keep pathogens and potentially harmful chemicals circulating in the blood from entering the brain and spinal cord. But that also makes it nearly impossible for many drugs and other molecules to be delivered to the brain via the bloodstream.
One variant of the AAV virus (credit: Jazzlw/Creative Commons)
The small, harmless adeno-associated virus (AAV) has been used to transport specific genes into the nuclei of cells; once there, the genes can be expressed (translated from DNA into proteins). AAVs can carry functional copies of genes to replace mutated forms present in individuals with genetic diseases. AAVs can also be used to deliver genes that provide instructions for generating molecules such as antibodies or fluorescent proteins that help researchers study, identify, and track certain cells.
Unfortunately, the blood-brain barrier has made it difficult to deliver AAVs and their genetic cargo to the central nervous system. So researchers have mostly relied on surgical injections, which deliver high concentrations of the virus at the injection site but little to the outlying areas.
That method is also extremely invasive. “One has to drill a hole through skull, then pierce tissue with a needle to the injection site,” explains Viviana Gradinaru, assistant professor of biology and biological engineering at Caltech and senior author on the paper. “The deeper the injection, the higher the risk of hemorrhage.”
Guradinaru says delivering via the BBB would avoid those problems, and “many disorders are not tightly localized. Neurodegenerative disorders like Huntington’s disease affect very large brain areas. Also, many complex behaviors are mediated by distributed interacting networks.”
Testing millions of self-replicating viral variants
In 2009, a group led by Brian Kaspar of Ohio State University published a paper, also in Nature Biotechnology, showing that an AAV strain called AAV9 injected into the bloodstream could make its way into the brain — but it was only efficient when used in neonatal (infant) mice. “The big challenge was how do we achieve the same efficiency in an adult,” says Gradinaru.
To do that efficiently, the researchers developed a high-throughput selection assay called CREATE (Cre REcombinase-based AAV Targeted Evolution) that allowed them to test millions of self-replicating viral variants of AAV in vivo simultaneously and to identify those that were best at entering the brain and delivering genes to a specific class of brain cells known as astrocytes.*
They also used PARS CLARITY, a technique developed previously in the Gradinaru lab to make normally opaque mammalian tissues transparent, allows organs to be examined without the laborious task of making thin slide-mounted sections. This allows researchers to more quickly screen the viral vectors for those that best target the cells and organs of interest.**
They narrowed it down to one variant, which they named AAV-PHP.B — it delivers genes to the brain and spinal cord efficiently through the vasculature into the brain (and to other tissues in the body).
In collaboration with colleagues from Stanford University, the researchers also showed that AAV-PHP.B is better than AAV9 at delivering genes to human neurons and glia.
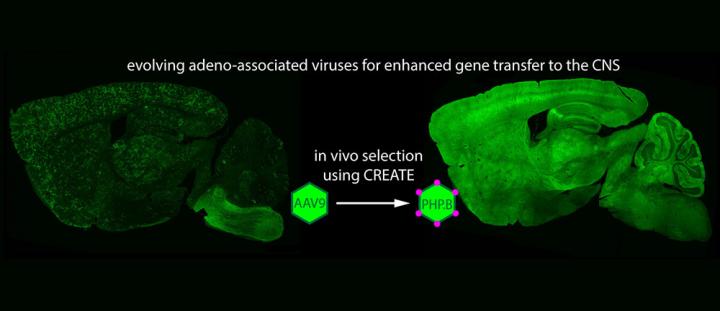
Using a new selection method, Caltech researchers have evolved the protein shell of a harmless virus, AAV9, so that it can more efficiently cross the blood brain barrier and deliver genes, such as the green fluorescent protein (GFP), to cells throughout the central nervous system. Here, GFP expression in naturally occurring AAV9 (left) can be seen distributed sparsely throughout the brain. The modified vector, AAV-PHP.B (right), provides more efficient GFP expression. (credit: Ben Deverman and the Gradinaru laboratory/Caltech)
The researchers hope to begin testing AAV-PHP.B’s ability to deliver potentially therapeutic genes in disease models. They are also working to further evolve the virus to make even better performing variants and to produce variants that target certain cell types with more specificity.
The Beckman Institute at Caltech recently opened a resource center called CLOVER (CLARITY, Optogenetics, and Vector Engineering Research Center) to support such research efforts involving tissue clearing and imaging, optogenetic studies, and custom gene-delivery vehicle development. Deverman is the center’s director, and Gradinaru is the principal investigator.
The work was supported by funding from the Hereditary Disease Foundation and the Caltech-City of Hope Biomedical Initiative, a National Institutes of Health (NIH) Director’s New Innovator Award, the NIH’s National Institute of Aging and National Institute of Mental Health, the Beckman Institute, and the Gordon and Betty Moore Foundation.
* They started with the AAV9 virus and modified a gene fragment that codes for a small loop on the surface of the capsid — the protein shell of the virus that envelops all of the virus’ genetic material. Using a common amplification technique, known as polymerase chain reaction (PCR), they created millions of viral variants. Each variant carried within it the genetic instructions to produce more capsids like itself.
Then they used their novel selection process to determine which variants most effectively delivered genes to astrocytes in the brain. Importantly, the new process relies on strategically positioning the gene encoding the capsid variants on the DNA strand between two short sequences of DNA, known as lox sites. These sites are recognized by an enzyme called Cre recombinase, which binds to them and inverts the genetic sequence between them. By injecting the modified viruses into transgenic mice that only express Cre recombinase in astrocytes, the researchers knew that any sequences flagged by the lox site inversion had successfully transferred their genetic cargo to the target cell type—here, astrocytes.
After one week, the researchers isolated DNA from brain and spinal cord tissue, and amplified the flagged sequences, thereby recovering only the variants that had entered astrocytes.
Next, they took those sequences and inserted them back into the modified viral genome to create a new library that could be injected into the same type of transgenic mice. After only two such rounds of injection and amplification, a handful of variants emerged as those that were best at crossing the blood-brain barrier and entering astrocytes.
“We went from millions of viruses to a handful of testable, potentially useful hits that we could go through systematically and see which ones emerged with desirable properties,” says Gradinaru.
Through this selection process, the researchers identified a variant dubbed AAV-PHP.B as a top performer. To test AAV-PHP.B, the researchers used it to deliver a gene that codes for a protein that glows green, making it easy to visualize which cells were expressing it. They injected the AAV-PHP.B or AAV9 (as a control) into different adult mice and after three weeks used the amount of green fluorescence to assess the efficacy with which the viruses entered the brain, the spinal cord, and the retina.
“We could see that AAV-PHP.B was expressed throughout the adult central nervous system with high efficiency in most cell types,” says Gradinaru. Indeed, compared to AAV9, AAV-PHP.B delivers genes to the brain and spinal cord at least 40 times more efficiently.
“What provides most of AAV-PHP.B’s benefit is its increased ability to get through the vasculature into the brain,” says Deverman. “Once there, many AAVs, including AAV9 are quite good at delivering genes to neurons and glia.”
** Making tissues transparent
Gradinaru notes that since AAV-PHP.B is delivered through the bloodstream, it reaches other parts of the body. “Although in this study we were focused on the brain, we were also able to use whole-body tissue clearing to look at its biodistribution throughout the body,” she says.
“In this case, the priority was to express the gene in the brain, but we can see by using whole-body clearing that you can actually have expression in many other organs and even in the peripheral nerves,” explains Gradinaru. “By making tissues transparent and looking through them, we can obtain more information about these viruses and identify targets that we might overlook otherwise.”
The biologists conducted follow-up studies up to a year after the initial injections and found that the protein continued to be expressed efficiently. Such long-term expression is important for gene therapy studies in humans.
Deverman says that the CREATE system could indeed be applied to develop AAVs capable of delivering genes specifically to many different cell types. “There are hundreds of different Cre transgenic lines available,” he says. “Researchers have put Cre recombinase under the control of gene regulatory elements so that it is only made in certain cell types. That means that regardless of whether your objective is to target liver cells or a particular type of neuron, you can almost always find a mouse that has Cre recombinase expressed in those cells.”
“The CREATE system gave us a good hit early on, but we are excited about the future potential of using this approach to generate viruses that have very good cell-type specificity in different organisms, especially the less genetically tractable ones,” says Gradinaru. “This is just the first step. We can take these tools and concepts in many exciting directions to further enhance this work, and we—with the Beckman Institute and collaborators—are ready to pursue those possibilities.”
Ben Deverman and the Gradinaru laboratory/Caltech and Nature Biotechnology | Green fluorescent protein (GFP) expression in the spinal cord of an adult mouse that received an intravenous injection of the modified virus, AAV-PHP.B, carrying the gene for GFP. The spinal cord sample was rendered transparent for imaging using a whole-body tissue clearing method called PARS CLARITY previously developed by the Gradinaru lab.
Abstract of Cre-dependent selection yields AAV variants for widespread gene transfer to the adult brain
Recombinant adeno-associated viruses (rAAVs) are commonly used vehicles for in vivogene transfer. However, the tropism repertoire of naturally occurring AAVs is limited, prompting a search for novel AAV capsids with desired characteristics. Here we describe a capsid selection method, called Cre recombination–based AAV targeted evolution (CREATE), that enables the development of AAV capsids that more efficiently transduce defined Cre-expressing cell populations in vivo. We use CREATE to generate AAV variants that efficiently and widely transduce the adult mouse central nervous system (CNS) after intravenous injection. One variant, AAV-PHP.B, transfers genes throughout the CNS with an efficiency that is at least 40-fold greater than that of the current standard, AAV9, and transduces the majority of astrocytes and neurons across multiple CNS regions. In vitro, it transduces human neurons and astrocytes more efficiently than does AAV9, demonstrating the potential of CREATE to produce customized AAV vectors for biomedical applications.
