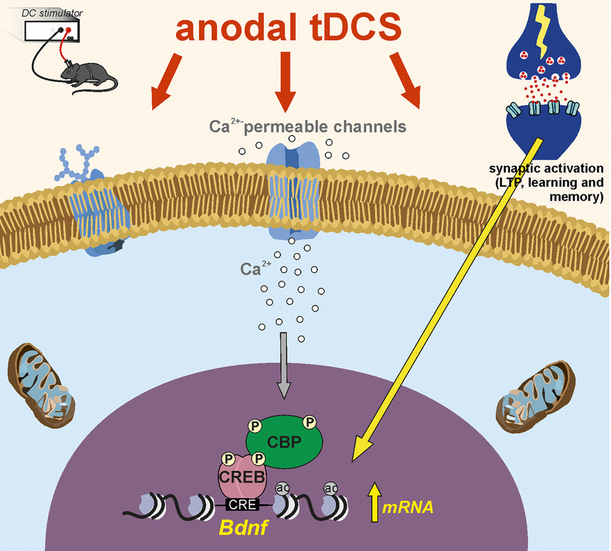
Transient increase in intracellular calcium ions during tDCS initiates molecular cascades leading to improved memory and brain plasticity* (credit: Maria Vittoria Podda et al./Scientific Reports)
Researchers at Catholic University Medical School in Rome have boosted the memory and mental performance of laboratory mice by transcranial Direct Current Stimulation (tDCS) and identified the molecular trigger for the improvement.
A noninvasive technique for brain stimulation, tDCS is applied using two small electrodes placed on the scalp, delivering short bursts of low-intensity electrical currents.
After exposing the mice to single 20-minute tDCS sessions, the researchers saw signs of improved memory and brain plasticity (the ability to form new connections between neurons when learning new information) in the hippocampus (a region of the brain critical to memory processing and storage), which lasted at least a week.
This boost was demonstrated by the enhanced performance of the mice during tests requiring them to navigate a water maze and distinguish between known and unknown objects.
Molecular trigger identified
The researchers also identified the molecular trigger behind the bolstered memory and plasticity: increased production of brain-derived neurotrophic factor (BDNF), a protein essential to brain growth that is synthesized naturally by neurons and is crucial to neuronal development and specialization.
The study was published in Nature Scientific Reports and sponsored by the Office of Naval Research (ONR) Global.
“In addition to potentially enhancing task performance for Sailors and Marines,” said ONR Global Commanding Officer Capt. Clark Troyer, “understanding how this technique works biochemically may lead to advances in the treatment of conditions like post-traumatic stress disorder, depression, and anxiety, which affect learning and memory in otherwise healthy individuals.”
The research may also have potential to strengthen learning and memory in both healthy people and those with cognitive deficits such as Alzheimer’s. “We already have promising results in animal models of Alzheimer’s disease,” said Claudio Grassi, PhD., who leads the research team.
Although tDCS has been used for years to treat patients suffering from conditions such as stroke, depression and bipolar disorder, there are few studies supporting a direct link between tDCS and improved plasticity, making Grassi’s work unique.
* Transient increase in intracellular Ca2+ during tDCS initiates molecular cascades leading to persistent changes in chromatin structure of brain-derived neurotrophic factor (BDNF). These include the phosphorylation of CREB, its binding to BDNF promoter I and recruitment of CREB/CREB-binding protein (CBP). CBP, in turn, promotes H3 acetylation at lysine 9 (H3K9ac) acetylation of BDNF (specifically at promoter I). As a result, stimuli such as long-term potentiation (LTP) induction protocol in slices or learning and memory in vivo are more effective in promoting transcription of BDNF previously primed by anodal tDCS.
Abstract of Anodal transcranial direct current stimulation boosts synaptic plasticity and memory in mice via epigenetic regulation of Bdnf expression
The effects of transcranial direct current stimulation (tDCS) on brain functions and the underlying molecular mechanisms are yet largely unknown. Here we report that mice subjected to 20-min anodal tDCS exhibited one-week lasting increases in hippocampal LTP, learning and memory. These effects were associated with enhanced: i) acetylation of brain-derived neurotrophic factor (Bdnf) promoter I; ii) expression of Bdnfexons I and IX; iii) Bdnf protein levels. The hippocampi of stimulated mice also exhibited enhanced CREB phosphorylation, pCREB binding to Bdnf promoter I and recruitment of CBP on the same regulatory sequence. Inhibition of acetylation and blockade of TrkB receptors hindered tDCS effects at molecular, electrophysiological and behavioral levels. Collectively, our findings suggest that anodal tDCS increases hippocampal LTP and memory via chromatin remodeling of Bdnf regulatory sequences leading to increased expression of this gene, and support the therapeutic potential of tDCS for brain diseases associated with impaired neuroplasticity.
