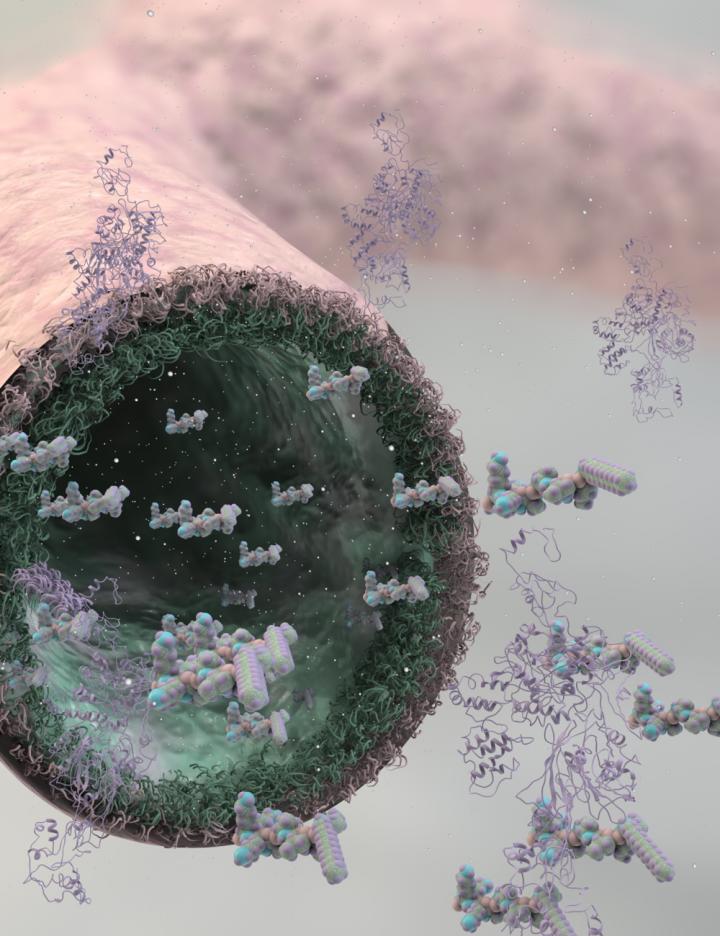
Researchers at Queen Mary University of London (QMUL) have developed a new bioinspired process using self-assembling organic molecules that can develop into complex tubular tissue-like structures. The process could lead to creating synthetic tissues that emulate veins, arteries, or even the blood-brain barrier, and that exhibit dynamic behaviors found in biological tissues like growth, morphogenesis, and healing.
The process uses solutions of peptide and protein molecules that self-assemble to form a dynamic tissue that can be guided to grow into complex shapes without the use of molds or techniques like 3-D printing.

Adipose-derived stem cells (mADSCs) seeded onto the protein/peptide membrane (credit: QMUL)
According to the researchers, the finding could allow scientists to study diseases such as Alzheimer’s with a high level of similarity to the real tissue and create better implants, complex tissues, and more effective drug-screening methods.
The study appeared September 28 in the journal Nature Chemistry. It has been partly funded by the European Research Council.
QMUL | Demonstrating a dynamic self-assembling protein-peptide membrane
Abstract of Co-assembly, spatiotemporal control and morphogenesis of a hybrid protein–peptide system
Controlling molecular interactions between bioinspired molecules can enable the development of new materials with higher complexity and innovative properties. Here we report on a dynamic system that emerges from the conformational modification of an elastin-like protein by peptide amphiphiles and with the capacity to access, and be maintained in, non-equilibrium for substantial periods of time. The system enables the formation of a robust membrane that displays controlled assembly and disassembly capabilities, adhesion and sealing to surfaces, self-healing and the capability to undergo morphogenesis into tubular structures with high spatiotemporal control. We use advanced microscopy along with turbidity and spectroscopic measurements to investigate the mechanism of assembly and its relation to the distinctive membrane architecture and the resulting dynamic properties. Using cell-culture experiments with endothelial and adipose-derived stem cells, we demonstrate the potential of this system to generate complex bioactive scaffolds for applications such as tissue engineering.