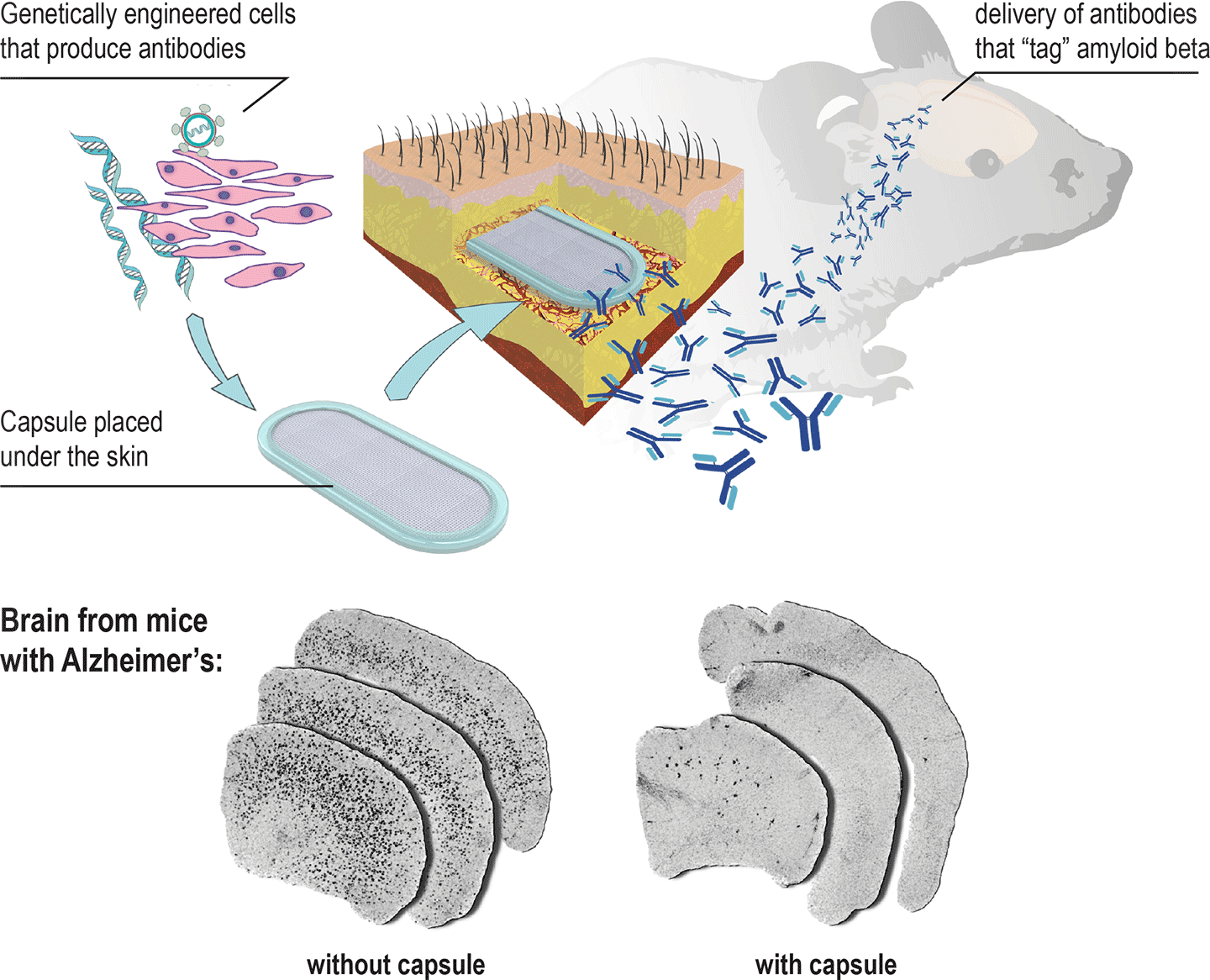
An implant that can prevent Alzheimer’s disease. A new capsule can be implanted under the skin to release antibodies that “tag” amyloid beta, signalling the patient’s immune system to clear it before it forms Alzheimer’s plaques. (credit: École polytechnique fédérale de Lausanne)
EPFL scientists have developed an implantable capsule containing genetically engineered cells that can recruit a patient’s immune system to combat Alzheimer’s disease.
Placed under the skin, the capsule releases antibody proteins that make their way to the brain and “tag” amyloid beta proteins, signalling the patient’s own immune system to attack and clear the amyloid beta proteins, which are toxic to neurons.
To be most effective, this treatment has to be given as early as possible, before the first signs of cognitive decline. Currently, this requires repeated vaccine injections, which can cause side effects. The new implant can deliver a steady, safe flow of antibodies.
Protection from immune-system rejection
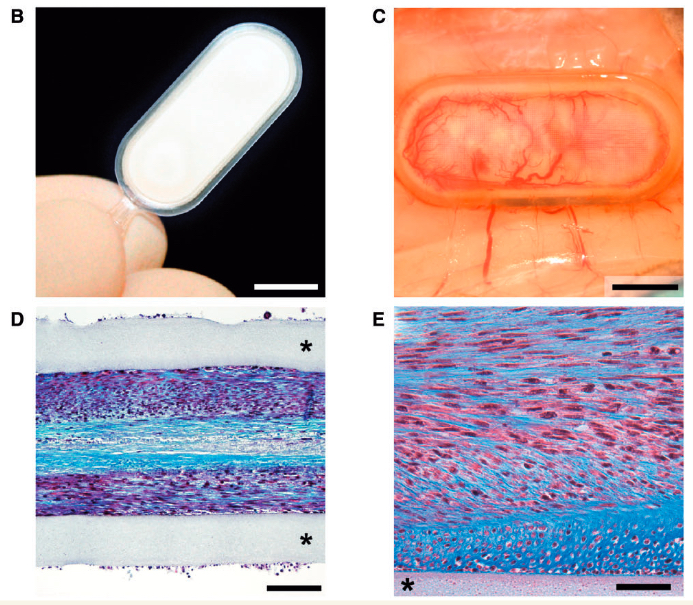
Cell encapsulation device for long-term subcutaneous therapeutic antibody delivery. (B) Macroscopic view of the encapsulation device, composed of a transparent frame supporting polymer permeable membranes and reinforced with an outer polyester mesh. (C) Dense neovascularization develops around a device containing antibody-secreting C2C12 myoblasts, 8 months after implantation in the mouse subcutaneous tissue. (D and E) Representative photomicrographs showing encapsulated antibody-secreting C2C12 myoblasts surviving at high density within the flat sheet device 39 weeks after implantation. (E) Higher magnification: note that the cells produce a collagen-rich matrix stained in blue with Masson’s trichrome protocol. Asterisk: polypropylene porous membrane. Scale bars = 750 mm (B and C),100 mm (D), 50 mm (E). (credit: Aurelien Lathuiliere et al./BRAIN)
The lab of Patrick Aebischer at EPFL designed the “macroencapsulation device” (capsule) with two permeable membranes sealed together with a polypropylene frame, containing a hydrogel that facilitates cell growth. All the materials used are biocompatible and the device is reproducible for large-scale manufacturing.
The cells of choice are taken from muscle tissue, and the permeable membranes let them interact with the surrounding tissue to get all the nutrients and molecules they need. The cells have to be compatible with the patient to avoid triggering the immune system against them, like a transplant can. To do that, the capsule’s membranes shield the cells from being identified and attacked by the immune system. This protection also means that cells from a single donor can be used on multiple patients.
The researchers tested the device mice in a genetic line commonly used to simulate Alzheimer’s disease over a course of 39 weeks, showing dramatic reduction of amyloid beta plaque load in the brain. The treatment also reduced the phosphorylation of the protein tau, another sign of Alzheimer’s observed in these mice.
“The proof-of-concept work demonstrates clearly that encapsulated cell implants can be used successfully and safely to deliver antibodies to treat Alzheimer’s disease and other neurodegenerative disorders that feature defective proteins,” according to the researchers.
The work is published in the journal BRAIN. It involved a collaboration between EPFL’s Neurodegenerative Studies Laboratory (Brain Mind Institute), the Swiss Light Source (Paul Scherrer Institute), and F. Hoffmann-La Roche. It was funded by the Swiss Commission for Technology and Innovation and F. Hoffmann-La Roche Ltd.
Abstract of A subcutaneous cellular implant for passive immunization against amyloid-β reduces brain amyloid and tau pathologies
Passive immunization against misfolded toxic proteins is a promising approach to treat neurodegenerative disorders. For effective immunotherapy against Alzheimer’s disease, recent clinical data indicate that monoclonal antibodies directed against the amyloid-β peptide should be administered before the onset of symptoms associated with irreversible brain damage. It is therefore critical to develop technologies for continuous antibody delivery applicable to disease prevention. Here, we addressed this question using a bioactive cellular implant to deliver recombinant anti-amyloid-β antibodies in the subcutaneous tissue. An encapsulating device permeable to macromolecules supports the long-term survival of myogenic cells over more than 10 months in immunocompetent allogeneic recipients. The encapsulated cells are genetically engineered to secrete high levels of anti-amyloid-β antibodies. Peripheral implantation leads to continuous antibody delivery to reach plasma levels that exceed 50 µg/ml. In a proof-of-concept study, we show that the recombinant antibodies produced by this system penetrate the brain and bind amyloid plaques in two mouse models of the Alzheimer’s pathology. When encapsulated cells are implanted before the onset of amyloid plaque deposition in TauPS2APP mice, chronic exposure to anti-amyloid-β antibodies dramatically reduces amyloid-β40 and amyloid-β42 levels in the brain, decreases amyloid plaque burden, and most notably, prevents phospho-tau pathology in the hippocampus. These results support the use of encapsulated cell implants for passive immunotherapy against the misfolded proteins, which accumulate in Alzheimer’s disease and other neurodegenerative disorders.
