Bioprinting a brain

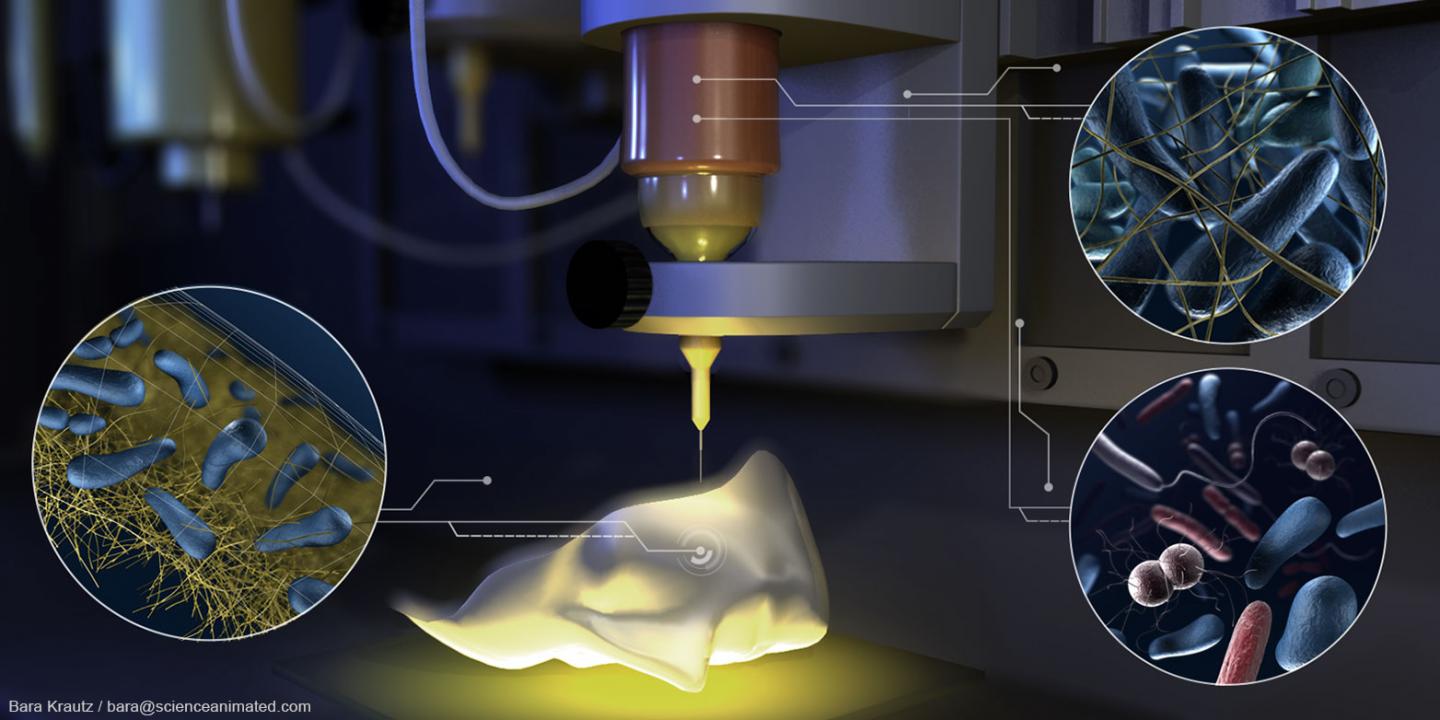
Cryogenic 3D-printing soft hydrogels. Top: the bioprinting process. Bottom: SEM image of general microstructure (scale bar: 100 µm). (credit: Z. Tan/Scientific Reports)
A new bioprinting technique combines cryogenics (freezing) and 3D printing to create geometrical structures that are as soft (and complex) as the most delicate body tissues — mimicking the mechanical properties of organs such as the brain and lungs.
The idea: “Seed” porous scaffolds that can act as a template for tissue regeneration (from neuronal cells, for example), where damaged tissues are encouraged to regrow — allowing the body to heal without tissue rejection or other problems. Using “pluripotent” stem cells that can change into different types of cells is also a possibility.
Smoothy. Solid carbon dioxide (dry ice) in an isopropanol bath is used to rapidly cool hydrogel ink (a rapid liquid-to-solid phase change) as it’s extruded, yogurt-smoothy-style. Once thawed, the gel is as soft as body tissues, but doesn’t collapse under its own weight — a previous problem.
Current structures produced with this technique are “organoids” a few centimeters in size. But the researchers hope to create replicas of actual body parts with complex geometrical structures — even whole organs. That could allow scientists to carry out experiments not possible on live subjects, or for use in medical training, replacing animal bodies for surgical training and simulations. Then on to mechanobiology and tissue engineering.
Source: Imperial College London, Scientific Reports (open-access).
How to generate electricity with your body

Bending a finger generates electricity in this prototype device. (credit: Guofeng Song et al./Nano Energy)
A new triboelectric nanogenerator (TENG) design, using a gold tab attached to your skin, will convert mechanical energy into electrical energy for future wearables and self-powered electronics. Just bend your finger or take a step.
Triboelectric charging occurs when certain materials become electrically charged after coming into contact with a different material. In this new design by University of Buffalo and Chinese scientists, when a stretched layer of gold is released, it crumples, creating what looks like a miniature mountain range. An applied force leads to friction between the gold layers and an interior PDMS layer, causing electrons to flow between the gold layers.
More power to you. Previous TENG designs have been difficult to manufacture (requiring complex lithography) or too expensive. The new 1.5-centimeters-long prototype generates a maximum of 124 volts but at only 10 microamps. It has a power density of 0.22 millwatts per square centimeter. The team plans larger pieces of gold to deliver more electricity and a portable battery.
Source: Nano Energy. Support: U.S. National Science Foundation, the National Basic Research Program of China, National Natural Science Foundation of China, Beijing Science and Technology Projects, Key Research Projects of the Frontier Science of the Chinese Academy of Sciences ,and National Key Research and Development Plan.
This artificial electrical eel may power your implants

How the eel’s electrical organs generate electricity by moving sodium (Na) and potassium (K) ions across a selective membrane. (credit: Caitlin Monney)
Taking it a giant (and a bit scary) step further, an artificial electric organ, inspired by the electric eel, could one day power your implanted implantable sensors, prosthetic devices, medication dispensers, augmented-reality contact lenses, and countless other gadgets. Unlike typical toxic batteries that need to be recharged, these systems are soft, flexible, transparent, and potentially biocompatible.
Doubles as a defibrillator? The system mimicks eels’ electrical organs, which use thousands of alternating compartments with excess potassium or sodium ions, separated by selective membranes. To create a jolt of electricity (600 volts at 1 ampere), an eel’s membranes allow the ions to flow together. The researchers built a similar system, but using sodium and chloride ions dissolved in a water-based hydrogel. It generates more than 100 volts, but at safe low current — just enough to power a small medical device like a pacemaker.
The researchers say the technology could also lead to using naturally occurring processes inside the body to generate electricity, a truly radical step.
Source: Nature, University of Fribourg, University of Michigan, University of California-San Diego. Funding: Air Force Office of Scientific Research, National Institutes of Health.
E-skin for Terminator wannabes

A section of “e-skin” (credit: Jianliang Xiao / University of Colorado Boulder)
A new type of thin, self-healing, translucent “electronic skin” (“e-skin,” which mimicks the properties of natural skin) has applications ranging from robotics and prosthetic development to better biomedical devices and human-computer interfaces.
Ready for a Terminator-style robot baby nurse? What makes this e-skin different and interesting is its embedded sensors, which can measure pressure, temperature, humidity and air flow. That makes it sensitive enough to let a robot take care of a baby, the University of Colorado mechanical engineers and chemists assure us. The skin is also rapidly self-healing (by reheating), as in The Terminator, using a mix of three commercially available compounds in ethanol.
The secret ingredient: A novel network polymer known as polyimine, which is fully recyclable at room temperature. Laced with silver nanoparticles, it can provide better mechanical strength, chemical stability and electrical conductivity. It’s also malleable, so by applying moderate heat and pressure, it can be easily conformed to complex, curved surfaces like human arms and robotic hands.
Source: University of Colorado, Science Advances (open-access). Funded in part by the National Science Foundation.
Altered Carbon
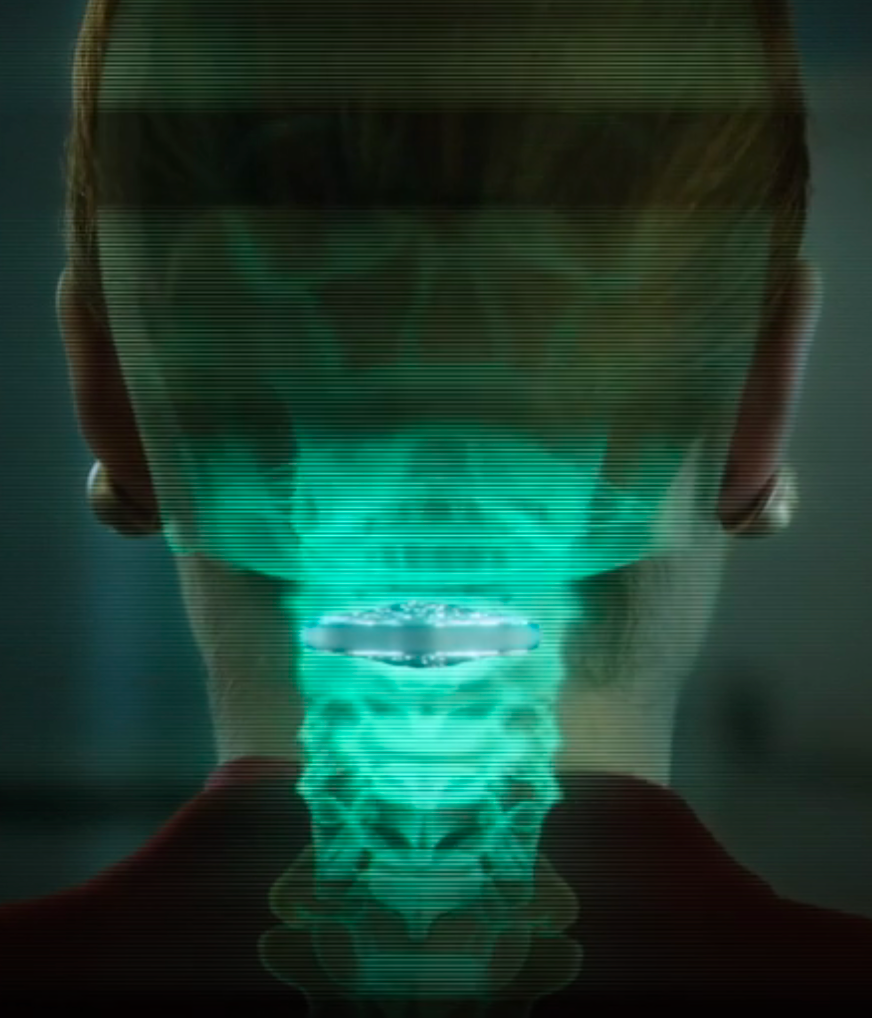
Vertebral cortical stack (credit: Netflix)
Altered Carbon takes place in the 25th century, when humankind has spread throughout the galaxy. After 250 years in cryonic suspension, a prisoner returns to life in a new body with one chance to win his freedom: by solving a mind-bending murder.
Resleeve your stack. Human consciousness can be digitized and downloaded into different bodies. A person’s memories have been encapsulated into “cortical stack” storage devices surgically inserted into the vertebrae at the back of the neck. Disposable physical bodies called “sleeves” can accept any stack.
But only the wealthy can acquire replacement bodies on a continual basis. The long-lived are called Meths, as in the Biblical figure Methuselah. The uber rich are also able to keep copies of their minds in remote storage, which they back up regularly, ensuring that even if their stack is destroyed, the stack can be resleeved (except for periods of time not backed up — as in the hack-murder).
Source: Netflix. Premiered on February 2, 2018. Based on the 2002 novel of the same title by Richard K. Morgan.