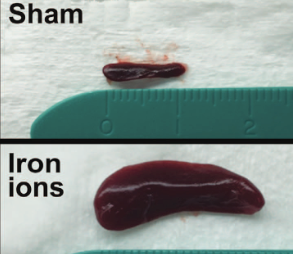
The spleen from a mouse exposed to a mission-relevant dose (20 cGy, 1 GeV/n) of iron ions (bottom) was ~ 30 times the normal volume compared with the spleen from a control mouse (top). (credit: C Rodman et al./Leukemia)
Radiation encountered in deep space travel may increase the risk of leukemia cancer in humans traveling to Mars, NASA-funded researchers at the Wake Forest Institute for Regenerative Medicine and colleagues have found, using mice transplanted with human stem cells.
“Our results are troubling because they show radiation exposure could potentially increase the risk of leukemia,” said Christopher Porada, Ph.D., associate professor of regenerative medicine and senior researcher on the project.
Radiation exposure is believed to be one of the most dangerous aspects of traveling to Mars, according to NASA. The average distance to Mars is 140 million miles, and a round trip could take three years.
The goal of the study, published in the journal Leukemia, was to assess the direct effects of simulated solar energetic particles (SEP) and galactic cosmic ray (GCR) radiation on human hematopoietic stem cells (HSCs). These stem cells comprise less than 0.1% of the bone marrow of adults, but produce the many types of blood cells that circulate through the body and work to transport oxygen, fight infection, and eliminate any malignant cells that arise.
For the study, human HSCs from healthy donors of typical astronaut age (30–55 years) were exposed to Mars mission-relevant doses of protons and iron ions — the same types of radiation that astronauts would be exposed to in deep space, followed by laboratory and animal studies to define the impact of the exposure.
“Radiation exposure at these levels was highly deleterious to HSC function, reducing their ability to produce almost all types of blood cells, often by 60–80 percent,” said Porada. “This could translate into a severely weakened immune system and anemia during prolonged missions in deep space.”
The radiation also caused mutations in genes involved in the hematopoietic process and dramatically reduced the ability of HSCs to give rise to mature blood cells.
Previous studies had already demonstrated that exposure to high doses of radiation, such as from X-rays, can have harmful (even life-threatening) effects on the body’s ability to make blood cells, and can significantly increase the likelihood of cancers, especially leukemias. However, the current study was the first to show a damaging effect of lower, mission-relevant doses of space radiation.
Mice develop T-cell acute lymphoblastic leukemia, weakened immune function
The next step was to assess how the cells would function in the human body. For that purpose, mice were transplanted with GCR-irradiated human HSCs, essentially “humanizing” the animals. The mice developed what appeared to be T-cell acute lymphoblastic leukemia — the first demonstration that exposure to space radiation may increase the risk of leukemia in humans.
“Our results show radiation exposure could potentially increase the risk of leukemia in two ways,” said Porada. “We found that genetic damage to HSCs directly led to leukemia. Secondly, radiation also altered the ability of HSCs to generate T and B cells, types of white blood cells involved in fighting foreign ‘invaders’ like infections or tumor cells. This may reduce the ability of the astronaut’s immune system to eliminate malignant cells that arise as a result of radiation-induced mutations.”
Porada said the findings are particularly troubling given previous work showing that conditions of weightlessness/microgravity present during spaceflight can also cause marked alterations in astronaut’s immune function, even after short duration missions in low-earth orbit, where they are largely protected from cosmic radiation.
Taken together, the results indicate that the combined exposure to microgravity and SEP/GCR radiation that would occur during extended deep space missions, such as to Mars, could potentially exacerbate the risk of immune-dysfunction and cancer,
NASA’s Human Research Program is also exploring conditions of microgravity, isolation and confinement, hostile and closed environments, and distance from Earth. The ultimate goal of the research is to make space missions as safe as possible.
Researchers at Wake Forest Baptist Medical Center, Brookhaven National Laboratory, and the University of California Davis Comprehensive Cancer Center were also involved in the study.
Abstract of In vitro and in vivo assessment of direct effects of simulated solar and galactic cosmic radiation on human hematopoietic stem/progenitor cells
Future deep space missions to Mars and near-Earth asteroids will expose astronauts to chronic solar energetic particles (SEP) and galactic cosmic ray (GCR) radiation, and likely one or more solar particle events (SPEs). Given the inherent radiosensitivity of hematopoietic cells and short latency period of leukemias, space radiation-induced hematopoietic damage poses a particular threat to astronauts on extended missions. We show that exposing human hematopoietic stem/progenitor cells (HSC) to extended mission-relevant doses of accelerated high-energy protons and iron ions leads to the following: (1) introduces mutations that are frequently located within genes involved in hematopoiesis and are distinct from those induced by γ-radiation; (2) markedly reduces in vitro colony formation; (3) markedly alters engraftment and lineage commitment in vivo; and (4) leads to the development, in vivo, of what appears to be T-ALL. Sequential exposure to protons and iron ions (as typically occurs in deep space) proved far more deleterious to HSC genome integrity and function than either particle species alone. Our results represent a critical step for more accurately estimating risks to the human hematopoietic system from space radiation, identifying and better defining molecular mechanisms by which space radiation impairs hematopoiesis and induces leukemogenesis, as well as for developing appropriately targeted countermeasures.