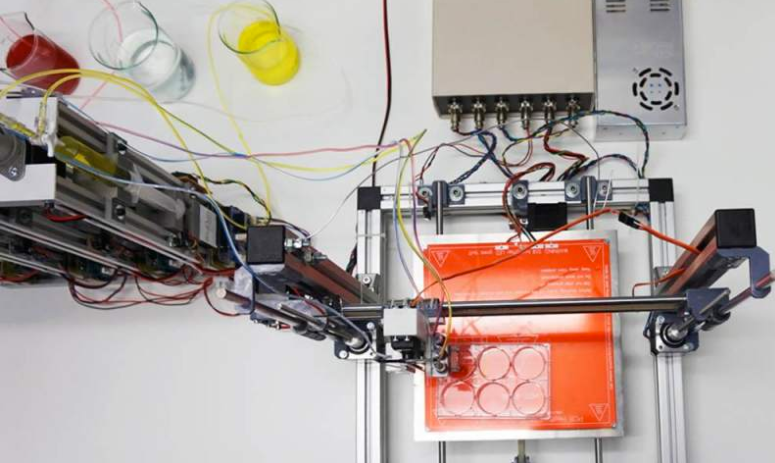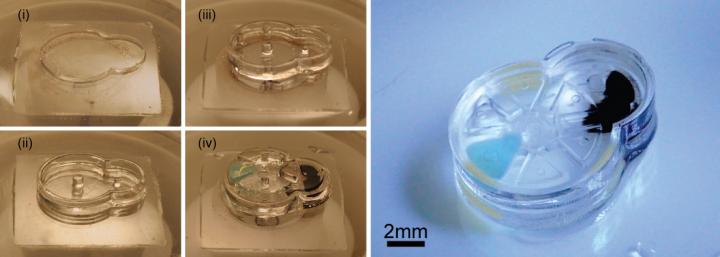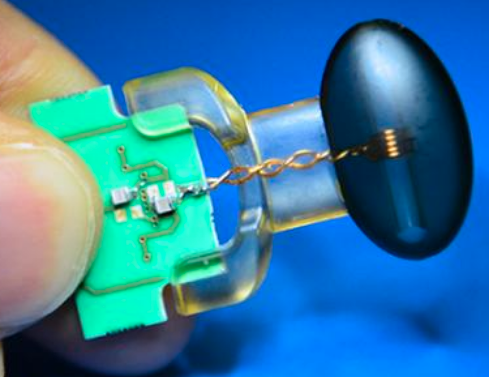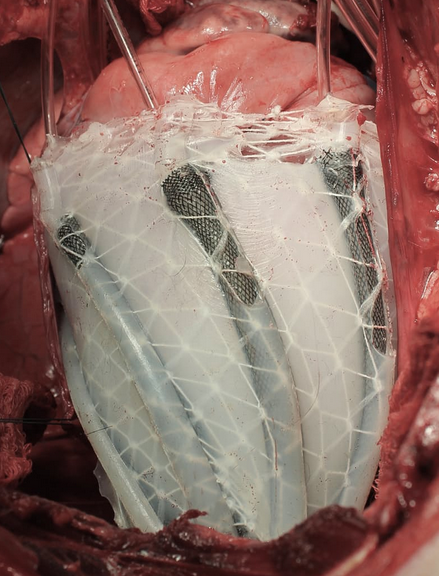
A soft robotic sleeve placed around the heart in a pig model of acute heart failure. The actuators embedded in the sleeve support heart function by mimicking the outer heart muscles that induce the heart to beat. (credit: Harvard SEAS)
An international team of scientists has developed a soft robotic sleeve that can be implanted on the external surface of the heart to restore blood circulation in pigs (and possibly humans in the future) whose hearts have stopped beating.
The device is a silicone-based system with two layers of actuators: one that squeezes circumferentially and one that squeezes diagonally, both designed to mimic the movement of healthy hearts when they beat.
Heart failure affects 41 million people worldwide. The concept of an artificial pump that aids cardiac function is not new and has been employed clinically with ventricular assist devices (VADs). “I’ve been implanting VADs in patients for a long time,” says Frank Pigula, MD, a former professor at Harvard and Boston Children’s Hospital who is co-corresponding author of an open-access paper published Jan. 18 in Science Translational Medicine. Pigula is now chief of pediatric cardiac surgery at Norton Children’s Hospital in Louisville, Kentucky.
The current-generation VADs systems directly expose a patient’s blood to artificial materials such as tubing and rotors. When blood touches a device’s components, it has a tendency to clot, which could lead to heart attacks, pulmonary embolism, strokes, or other complications, Pigula explained. To prevent clots from forming, it’s necessary to use blood thinning and anticoagulatory medications such as heparin and warfarin, which are complication-prone.
How to make a soft robotic sleeve for a human heart
That’s where the soft robotic sleeve developed by Pigula’s team comes in.
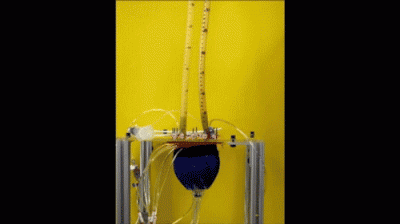
In vitro demonstration of the pumping of the soft robotic sleeve (credit: Ellen Roche/Harvard University)
The robotic sleeve can be implanted on the external surface of the heart, preventing circulating blood from ever coming in contact with the device’s components, possibly eliminating or decreasing the need for anticoagulatory drugs.
In designing the soft robotic sleeve, the researchers were inspired by the structure and movement of the heart itself. The two actuators are formed in thin layers of silicone that are layered on each other in roughly the same orientation as the muscle fibers in the heart. The sleeve is about half a millimeter thick, which is about the width of 5–10 human hairs.
To adhere the sleeve to pig hearts, the researchers used an FDA-approved adherent on the apex, or tip, of the heart. However, the researchers found this caused severe inflammation at the point of adherence, which could interfere with the ability to implant the sleeve for long periods of time. So the researchers employed a gel to adhere the sleeve to the heart, a technique that lessened the inflammation.
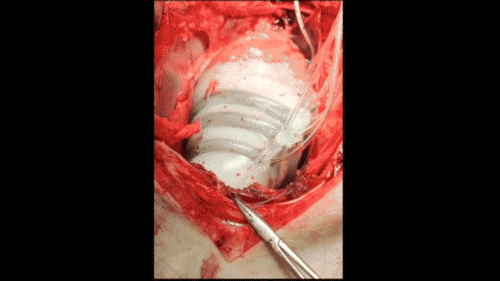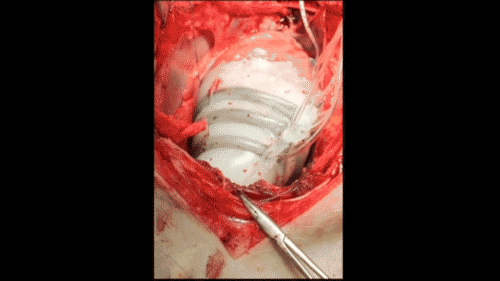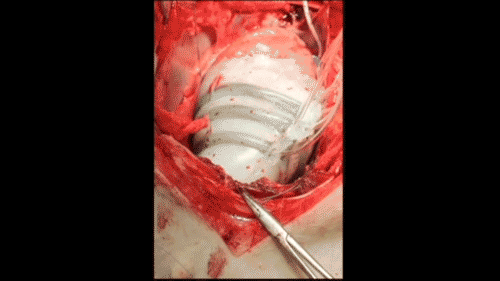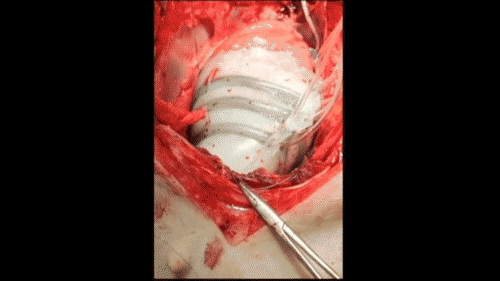
In vivo demonstration of cardiac assist in a porcine model of acute heart failure (credit: Ellen Rouche/Harvard SEAS)
To test the sleeves, the scientists implanted them on the hearts of pigs and induced acute heart failure, resulting in a 50–60% drop in cardiac output. Turning on the sleeve restored 97% of the original cardiac output.
“The soft robotic actuators are essentially artificial muscles,” says Nikolay Vasilyev, MD, a staff scientist in cardiac surgery research at Boston Children’s Hospital and co-author on the recent study. “In this sense, the robotic sleeve mimics both ventricles of the heart.”
The soft robotic heart sleeve also contains sophisticated sensing abilities that measure pressure at specific points on the heart’s surface.
“This work represents an exciting proof-of-concept result for this soft robot, demonstrating that it can safely interact with soft tissue and lead to improvements in cardiac function,” said Conor Walsh, senior author of the paper and the John L. Loeb Associate Professor of Engineering and Applied Sciences at SEAS and Core Faculty Member at Harvard’s Wyss Institute. “We envision many other future applications where such devices can deliver mechanotherapy both inside and outside of the body.”
However, “the human body is remarkably good at detecting foreign materials and mounting immune responses to them, so it will be tricky business to find a biologically inert material that will not, over the long run, scar the tissues it’s physically associated with,” according to a physician who was not involved in the research. “This is less critical for non-vital organs like soft-tissue silicone implants, but a thin layer of scar tissue around the heart could have serious implications for the stiffness, structural integrity, and function of native heart tissue.”
The research was a collaboration between Harvard’s SEAS and Wyss Institute, Boston Children’s Hospital, National University of Ireland, Technische Universität München, Boston Children’s Hospital, University of Leeds, University of Central Florida, Royal College of Surgeons in Ireland, Trinity College Dublin, UCLA, and University of Louisville.
The work was supported by the Translational Research Program grant from Boston Children’s Hospital, a Director’s Challenge Cross-Platform grant from the Wyss Institute for Biologically Inspired Engineering, Harvard School of Engineering and Applied Sciences, and Science Foundation Ireland.
Abstract of Soft robotic sleeve supports heart function
There is much interest in form-fitting, low-modulus, implantable devices or soft robots that can mimic or assist in complex biological functions such as the contraction of heart muscle. We present a soft robotic sleeve that is implanted around the heart and actively compresses and twists to act as a cardiac ventricular assist device. The sleeve does not contact blood, obviating the need for anticoagulation therapy or blood thinners, and reduces complications with current ventricular assist devices, such as clotting and infection. Our approach used a biologically inspired design to orient individual contracting elements or actuators in a layered helical and circumferential fashion, mimicking the orientation of the outer two muscle layers of the mammalian heart. The resulting implantable soft robot mimicked the form and function of the native heart, with a stiffness value of the same order of magnitude as that of the heart tissue. We demonstrated feasibility of this soft sleeve device for supporting heart function in a porcine model of acute heart failure. The soft robotic sleeve can be customized to patient-specific needs and may have the potential to act as a bridge to transplant for patients with heart failure.