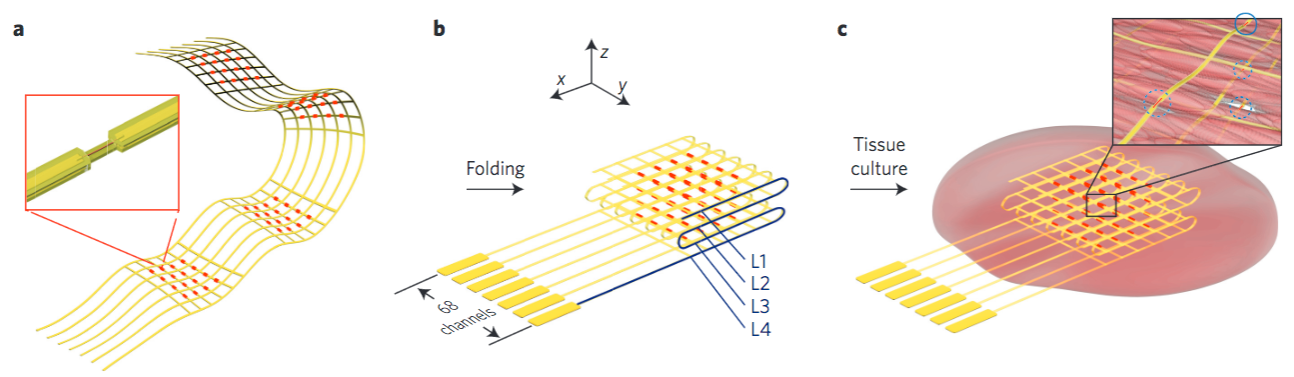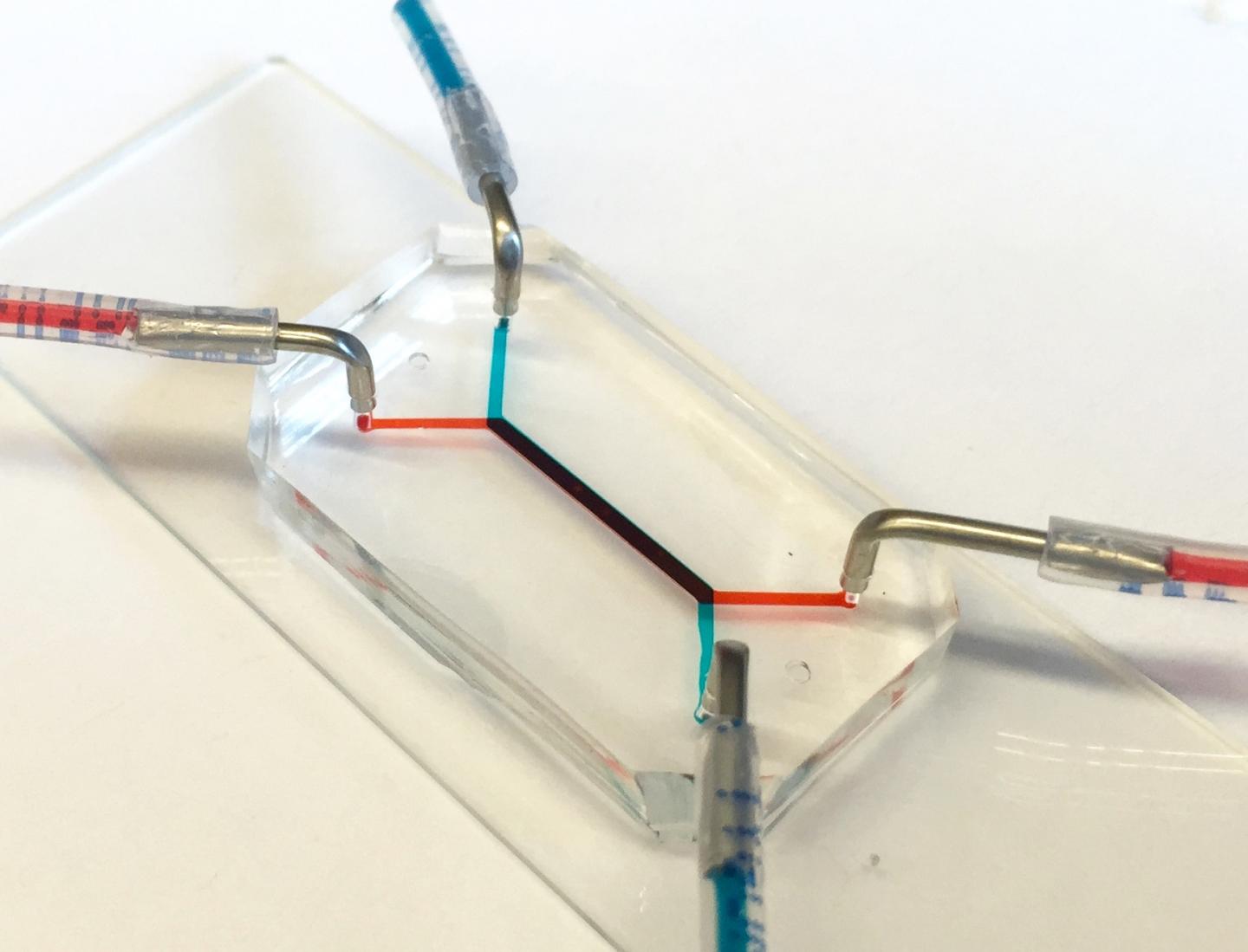
The flash-drive-sized device contains two layers of human cells that model the interface between mother and fetus. Microfluidic channels on both sides of those layers allow researchers to study how molecules are transported through, or are blocked by, that interface. (credit: University of Pennsylvania)
Researchers at the University of Pennsylvania have developed the first placenta-on-a-chip that can fully model the transport of nutrients across the placental barrier — part of a nationwide effort sponsored by the March of Dimes to identify causes of preterm birth and ways to prevent it.
Prematurely born babies may experience lifelong, debilitating consequences, but the underlying mechanisms of this condition are not well understood due in part to the difficulties of experimenting with intact, living human placentae.
Like other organs-on-chips, such as ones developed to simulate lungs, intestines, and eyes, the placenta-on-a-chip provides a unique capability to mimic and study the function of that human organ in ways that have not been possible using traditional tools, which are limited by complexity, the scarcity of samples, and the limited lifespan of how long the tissue remains viable (for only a few hours after delivery), according to the researchers.
Modeling the vital placental barrier
The flash-drive-sized device contains two layers of human cells that model the interface between mother and fetus. Microfluidic channels on either side of those layers allow researchers to study how molecules are transported through, or are blocked by, that interface.
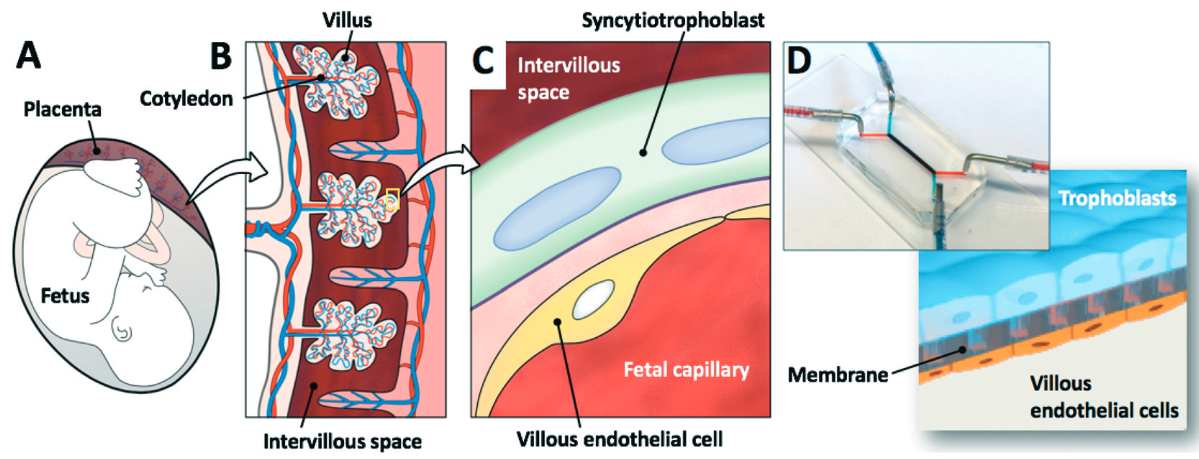
(A) Schematic of a human fetus and placenta within the uterine cavity. (B) Cross-sectional view of the placenta showing villi (vascular projections that increase the surface area of a membrane) in direct contact with maternal blood in (C),where the maternal intervillous space is separated from the fetal capillary by the maternal–fetal interface, which is composed of the syncytiotrophoblast, basal lamina, and villous endothelial cells. (D) 3-D microarchitecture of the placental barrier reconstituted within the placenta-on-a-chip, which consists of upper and lower microchannels separated by a thin, semipermeable membrane. Trophoblast cells are cultured in the upper microchannel layer and villous endothelial cells are grown in the lower microchannel layer. (credit: Cassidy Blundell et al./Lab on a Chip)
The researchers’ placenta-on-a-chip is a clear silicone device with two parallel microfluidic channels separated by a porous membrane. On one side of those pores, trophoblast cells, which are found at the placental interface with maternal blood, are grown. On the other side are endothelial cells, found on the interior of fetal blood vessels.
The layers of those two cell types mimic the placental barrier, the gatekeeper (or filter) that controls flow between the maternal and fetal circulatory systems, including nutrients that must pass, but also foreign agents like viruses that must be blocked.
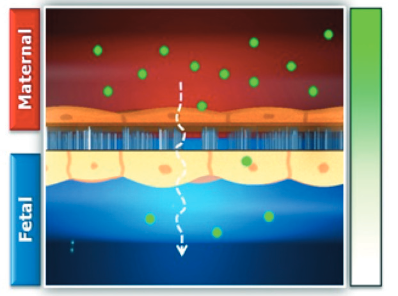
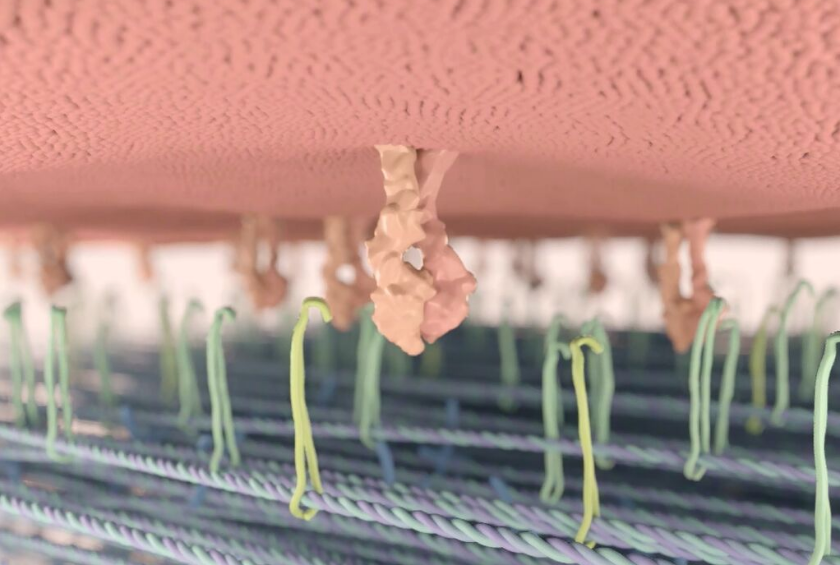
A concentration gradient of glucose (green dots) is generated across the microengineered placental barrier to drive glucose transport from the maternal to fetal compartments. (credit: Cassidy Blundell et al./Lab on a Chip)
In their new study, the Penn researchers have demonstrated that the two layers of cells continue to grow and develop while inside the chip, undergoing a process known as “syncytialization.”
“The placental cells change over the course of pregnancy,” explained Dan Huh, the Wilf Family Term Assistant Professor of Bioengineering in Penn’s School of Engineering and Applied Science. “During pregnancy, the placental trophoblast cells actually fuse with one another to form an interesting tissue called syncytium. The barrier also becomes thinner as the pregnancy progresses, and with our new model we’re able to reproduce this change.
“This process is very important because it affects placental transport and was a critical aspect not represented in our previous model.”
The Penn team validated the new model by showing glucose transfer rates across this syncytialized barrier matched those measured in perfusion studies of donated human placentae.
“The placenta is arguably the least understood organ in the human body,” Huh said, “and much remains to be learned about how transport between mother and fetus works at the tissue, cellular and molecular levels. An isolated whole organ is an not ideal platform for these types of mechanistic studies.”
Studies of preterm birth
While the placenta-on-a-chip is still in the early stages of testing, researchers at Penn and beyond are already planning to use it in studies on preterm birth. The rate of preterm birth is still about 10 to 11 percent of all pregnancies.
“Eventually,” Huh said, “we hope to leverage the unique capabilities of our model to demonstrate the potential of organ-on-a-chip technology as a new strategy to innovate basic and translational research in reproductive biology and medicine.”
The study was published in the journal Lab on a Chip. The research was supported by the March of Dimes Prematurity Research Center at the University of Pennsylvania and the National Institutes of Health.
Abstract of A microphysiological model of the human placental barrier
During human pregnancy, the fetal circulation is separated from maternal blood in the placenta by two cell layers – the fetal capillary endothelium and placental trophoblast. This placental barrier plays an essential role in fetal development and health by tightly regulating the exchange of endogenous and exogenous materials between the mother and the fetus. Here we present a microengineered device that provides a novel platform to mimic the structural and functional complexity of this specialized tissue in vitro. Our model is created in a multilayered microfluidic system that enables co-culture of human trophoblast cells and human fetal endothelial cells in a physiologically relevant spatial arrangement to replicate the characteristic architecture of the human placental barrier. We have engineered this co-culture model to induce progressive fusion of trophoblast cells and to form a syncytialized epithelium that resembles the syncytiotrophoblast in vivo. Our system also allows the cultured trophoblasts to form dense microvilli under dynamic flow conditions and to reconstitute expression and physiological localization of membrane transport proteins, such as glucose transporters (GLUTs), critical to the barrier function of the placenta. To provide a proof-of-principle for using this microdevice to recapitulate native function of the placental barrier, we demonstrated physiological transport of glucose across the microengineered maternal–fetal interface. Importantly, the rate of maternal-to-fetal glucose transfer in this system closely approximated that measured in ex vivo perfused human placentas. Our “placenta-on-a-chip” platform represents an important advance in the development of new technologies to model and study the physiological complexity of the human placenta for a wide variety of applications.