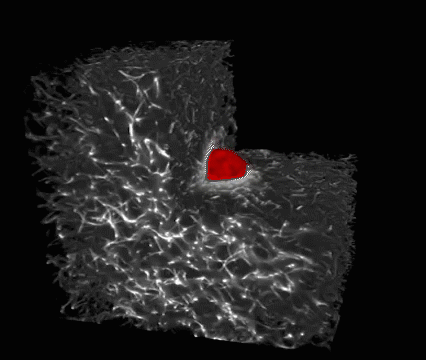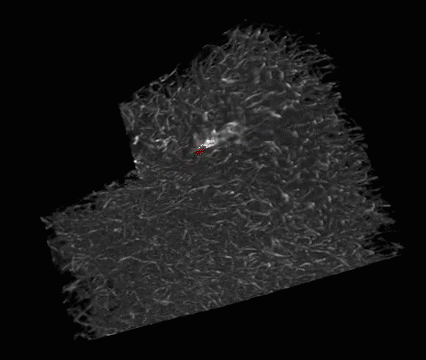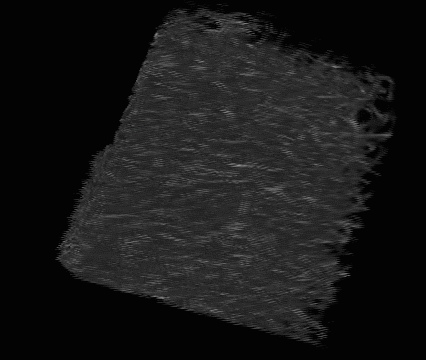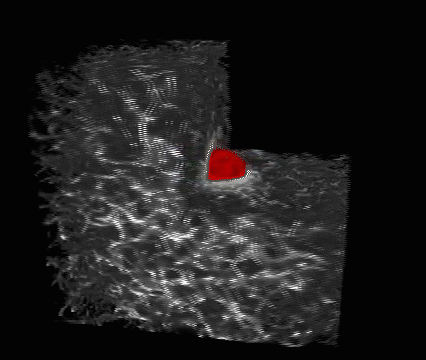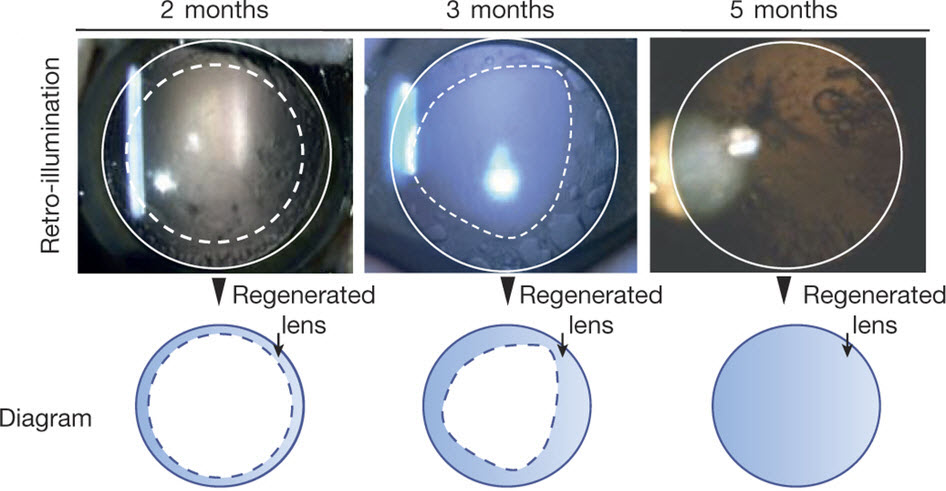
Lens regeneration in monkeys after minimally invasive surgery. Slit-lamp microscopy showed regenerating lens tissue grew from the peripheral to the central lens in a circular symmetrical pattern 2–3 months after surgery, reaching the center at 5 months post-surgery; direct illumination showed that the visual axis remained translucent. (credit: Haotian Lin et al./Nature)
Researchers at University of California, San Diego School of Medicine and Shiley Eye Institute, with colleagues in China, have developed an eye lens restoration treatment that has been tested in monkeys and in a small human clinical trial. It produced much fewer surgical complications than the current standard-of-care and resulted in regenerated lenses with superior visual function in all 12 of the pediatric cataract patients who received the new surgery.
Congenital cataracts — lens clouding that occurs at birth or shortly thereafter — is a significant cause of blindness in children.
Using the patients’ own stem cells
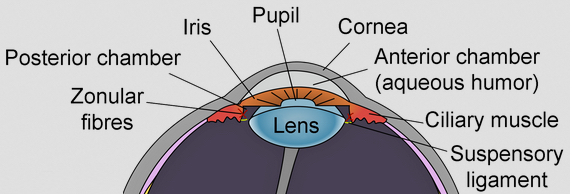
Eye lens (credit: Wikimedia Commons)
In the new research, Kang Zhang*, MD, PhD and colleagues relied on the regenerative potential of the patients’ own lens epithelial stem cells (LECs) at the site of the injury or problem, instead of creating stem cells in the lab and introducing them back into the patient (with potential hurdles like pathogen transmission and immune rejection).
After confirming the regenerative potential of LECs in animal models, the researchers developed a novel minimally invasive surgery method that preserves the integrity of the lens capsule — a membrane that helps give the lens its required shape to function — and also developed a way to stimulate LECs to grow and form a new lens with vision.
They found the new surgical technique allowed pre-existing LECs to regenerate functional lenses, producing a clear, regenerated biconvex lens in all of the patients’ eyes after three months.
Age-related cataracts next
Zhang said he and colleagues are now looking to expand their work to treating age-related cataracts. Age-related cataracts is the leading cause of blindness in the world. More than 20 million Americans suffer from cataracts, and more than 4 million surgeries are performed annually to replace the clouded lens with an artificial plastic version, called an intraocular lens.
Despite technical advances, a large portion of patients undergoing surgery are left with suboptimal vision post-surgery and are dependent upon corrective eyewear for driving a car and/or reading a book. “We believe that our new approach will result in a paradigm shift in cataract surgery and may offer patients a safer and better treatment option in the future.”
The findings are published in the March 9 online issue of Nature. Co-authors on the study include scientists at UC San Diego; Sichuan University, China; Guangzhou KangRui Biological Pharmaceutical Technology Company, China; and University of Texas Southwestern Medical Center.
Funding for this research came, in part, from the 973 Program (National Basic Research Program of China); a Major International Joint Research Project; 863 Program (State High-Tech Development Plan of China); the National Natural Science Foundation of China; the State Key Laboratory of Ophthalmology, Zhongshan Ophthalmic Center, Sun Yatsen University; Research to Prevent Blindness; and the Howard Hughes Medical Institute.
* Chief of Ophthalmic Genetics, founding director of the Institute for Genomic Medicine and co-director of Biomaterials and Tissue Engineering at the Institute of Engineering in Medicine, both at UC San Diego School of Medicine.
Abstract of Lens regeneration using endogenous stem cells with gain of visual function
The repair and regeneration of tissues using endogenous stem cells represents an ultimate goal in regenerative medicine. To our knowledge, human lens regeneration has not yet been demonstrated. Currently, the only treatment for cataracts, the leading cause of blindness worldwide, is to extract the cataractous lens and implant an artificial intraocular lens. However, this procedure poses notable risks of complications. Here we isolate lens epithelial stem/progenitor cells (LECs) in mammals and show that Pax6 and Bmi1 are required for LEC renewal. We design a surgical method of cataract removal that preserves endogenous LECs and achieves functional lens regeneration in rabbits and macaques, as well as in human infants with cataracts. Our method differs conceptually from current practice, as it preserves endogenous LECs and their natural environment maximally, and regenerates lenses with visual function. Our approach demonstrates a novel treatment strategy for cataracts and provides a new paradigm for tissue regeneration using endogenous stem cells.