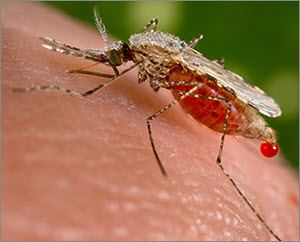
Wyss Institute scientists believe that synthetic gene drives, if researched responsibly, might be used in the future to render mosquito populations unable to transmit malaria (credit: CDC)
An international group of 26 experts, including prominent genetic engineers and fruit fly geneticists, has unanimously recommended a series of preemptive measures to safeguard gene drive research from accidental (or intentional) release from laboratories.
RNA-guided gene drives are genetic elements — found naturally in the genomes of most of the world’s organisms — that increase the chance of the gene they carry being passed on to all offspring. So they can quickly spread through populations if not controlled.
Looking to these natural systems, researchers around the world, including some scientists, are developing synthetic gene drives that could one day be leveraged by humans to purposefully alter the traits of wild populations of organisms to prevent disease transmission and eradicate invasive species.
What could possibly go wrong?
These synthetic gene drives, designed using an RNA-guided gene editing system called CRISPR, could one day improve human health and the environment by preventing mosquitoes and ticks from spreading diseases such as malaria and Lyme; by promoting sustainable agriculture through control of crop pests without the use of toxic pesticides and herbicides; and by protecting at-risk ecosystems from the spread of destructive, invasive species such as rats or cane toads.

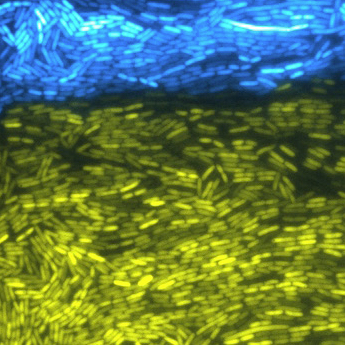
Most genome alterations don’t persist in nature. Only 50 percent of transgenic mosquito offspring (left) will carry the altered gene, so it may persist at low frequency or go instinct. With gene drive (right), using CRISPR/Cas9, all of the offspring will carry the altered gene and will be inherited through the population. (credit: adapted from Wyss Institute video)
However, the development of RNA-guided gene drive technology calls for enhanced safety measures. That’s because its capability to also affect shared ecosystems if organisms containing synthetic gene drives are accidentally or deliberately released from a laboratory. This potential risk is especially relevant with highly mobile species such as fruit flies or mosquitoes.
Guidelines available
“One of the great successes of engineering is the development of safety features, such as the rounding of sharp corners on objects and the invention of airbags for cars, and in biological engineering we want to emulate the process of designing safety features in ways relevant to the technologies we develop,” said Wyss Core Faculty member George Church, Ph.D., who leads the Synthetic Biology Platform at the Wyss Institute. Church is also the Robert Winthrop Professor of Genetics at Harvard Medical School and Professor of Health Sciences and Technology at Harvard and MIT.
At the Wyss Institute, enhanced protocols for safely and securely researching emerging biotechnologies, including RNA–guided gene drives, have already been formally implemented. The safeguards were put in place proactively, step–by–step, in direct parallel with the development of the first RNA-guided gene drives at the Wyss Institute.
The working documents have been made publicly available by the Institute to encourage widespread adoption of multi-tier confinement and risk assessment procedures. Church was instrumental in the design of the enhanced biosafety and biosecurity protocols.
Now, research teams from the Wyss Institute and University of California, San Diego — the only two groups to have published work on RNA-guided CRISPR gene drives — have proactively assembled an international group of 26 experts, including prominent genetic engineers and fruit fly geneticists, to unanimously recommend a series of preemptive measures to safeguard gene drive research.
Open-access research recommended
Led by Wyss Institute Technology Development Fellow, Kevin Esvelt, Ph.D., and UC San Diego Professor of Cell and Developmental Biology Ethan Bier, Ph.D., the 26 authors of this consensus recommendation, which is published online in Science Express journal and includes representatives from every major group known to be working on gene drives, calls for all researchers to use multiple confinement strategies in order to prevent the accidental alteration of wild populations.
The group also provides explicit recommended guidelines for regulatory authorities evaluating proposed new work. And Esvelt and others are hopeful that the field of gene drive research is so nascent that it may be possible to build a community of scientists that share their research with the public throughout the development process.
“This would promote collaboration and avoid needless duplication of efforts among different research groups while allowing diverse voices to help guide the development of a technology that could improve our shared world,” said Esvelt. “And eventually, it might inspire a similar shift towards full transparency in other scientific fields of collective public importance.”
“The scientific community has a responsibility to the public and to the environment to constantly assess how new biotechnologies could potentially impact our world,” said Wyss Institute Founding Director Donald E. Ingber, M.D., Ph.D.
“This proactive consensus recommendation — reached in an extraordinary demonstration of the power of scientific collaboration over competition — provides concrete, useful guidelines for safeguarding our shared ecosystem while ensuring that remarkable breakthroughs, such as synthetic gene drives, can be applied to their full potential for the greater good.”
Wyss Institute at Harvard University | CRISPER-Cas9: Gene Drive
This animation explains how gene drives could one day be used to spread gene alterations through targeted wild populations over many generations, for purposes such as preventing spread of insect-borne disease and controlling invasive plant species. To ensure gene drives have the potential to be used for the greater good in the future, Wyss Institute Technology Development Fellow Kevin Esvelt, Ph.D., has co-led an international consensus of 26 scientists to recommend safeguards to prevent synthetic gene drive research from having any accidental impacts on the world’s shared ecosystems.
Abstract of A mucosal vaccine against Chlamydia trachomatis generates two waves of protective memory T cells
INTRODUCTION: Administering vaccines through nonmucosal routes often leads to poor protection against mucosal pathogens, presumably because such vaccines do not generate memory lymphocytes that migrate to mucosal surfaces. Although mucosal vaccination induces mucosa-tropic memory lymphocytes, few mucosal vaccines are used clinically; live vaccine vectors pose safety risks, whereas killed pathogens or molecular antigens are usually weak immunogens when applied to intact mucosa. Adjuvants can boost immunogenicity; however, most conventional mucosal adjuvants have unfavorable safety profiles. Moreover, the immune mechanisms of protection against many mucosal infections are poorly understood.
RATIONALE: One case in point is Chlamydia trachomatis (Ct), a sexually transmitted intracellular bacterium that infects >100 million people annually. Mucosal Ct infections can cause female infertility and ectopic pregnancies. Ct is also the leading cause of preventable blindness in developing countries and induces pneumonia in infants. No approved vaccines exist to date. Here, we describe a Ct vaccine composed of ultraviolet light–inactivated Ct (UV-Ct) conjugated to charge-switching synthetic adjuvant nanoparticles (cSAPs). After immunizing mice with live Ct, UV-Ct, or UV-Ct–cSAP conjugates, we characterized mucosal immune responses to uterine Ct rechallenge and dissected the underlying cellular mechanisms.
RESULTS: In previously uninfected mice, Ct infection induced protective immunity that depended on CD4 T cells producing the cytokine interferon-γ, whereas uterine exposure to UV-Ct generated tolerogenic Ct-specific regulatory T cells, resulting in exacerbated bacterial burden upon Ct rechallenge. In contrast, mucosal immunization with UV-Ct–cSAP elicited long-lived protection. This differential effect of UV-Ct–cSAP versus UV-Ct was because the former was presented by immunogenic CD11b+CD103– dendritic cells (DCs), whereas the latter was presented by tolerogenic CD11b–CD103+ DCs. Intrauterine or intranasal vaccination, but not subcutaneous vaccination, induced genital protection in both conventional and humanized mice. Regardless of vaccination route, UV-Ct–cSAP always evoked a robust systemic memory T cell response. However, only mucosal vaccination induced a wave of effector T cells that seeded the uterine mucosa during the first week after vaccination and established resident memory T cells (TRM cells). Without TRM cells, mice were suboptimally protected, even when circulating memory cells were abundant. Optimal Ct clearance required both early uterine seeding by TRM cells and infection-induced recruitment of a second wave of circulating memory cells.
CONCLUSIONS: Mucosal exposure to both live Ct and inactivated UV-Ct induces antigen-specific CD4 T cell responses. While immunogenic DCs present the former to promote immunity, the latter is instead targeted to tolerogenic DCs that exacerbate host susceptibility to Ct infection. By combining UV-Ct with cSAP nanocarriers, we have redirected noninfectious UV-Ct to immunogenic DCs and achieved long-lived protection. This protective vaccine effect depended on the synergistic action of two memory T cell subsets with distinct differentiation kinetics and migratory properties. The cSAP technology offers a platform for efficient mucosal immunization that may also be applicable to other mucosal pathogens.