2016 Rio Games Opening Ceremony promo (credit: NBC)
A week ago on KurzweilAI, we learned that prolonged sitting may increase risk of death, but that an hour of moderate exercise a day is enough to counter health risks. Now new research suggests that such exercise results in larger brain size and lowered dementia risk, while other new research suggests that the new neurons created in that exercise preserve old memories, contrary to previous research.
Exercise results in larger brain size and lowered dementia risk
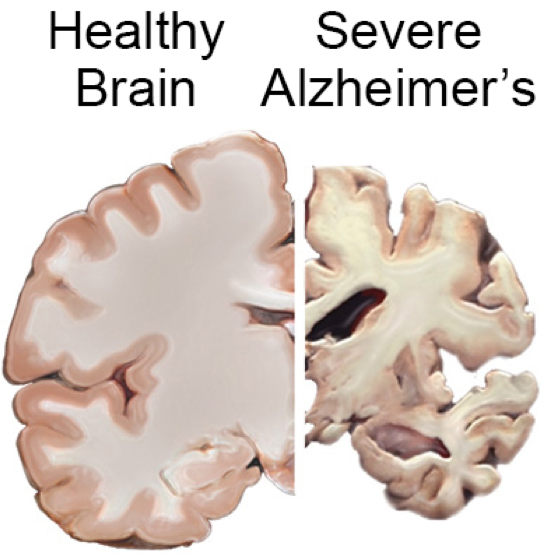
UCLA researchers have found an association between low physical activity and a higher risk for dementia in older individuals, based on data from the landmark Framingham Heart Study.
The researchers found that physical activity particularly affected the size of the hippocampus, involved in short-term memory. They also found the protective effect of regular physical activity against dementia was strongest in people age 75 and older.
This suggests that regular physical activity for older adults could lead to higher brain volumes and a reduced risk for developing dementia.
The Framingham study was begun in 1948 primarily as a way to trace factors and characteristics leading to cardiovascular disease, but also examining dementia and other physiological conditions. For this study, the UCLA researchers followed an older, community-based cohort from the Framingham study for more than a decade to examine the association between physical activity and the risk for incident dementia and subclinical brain MRI markers of dementia.
The study appears in the Journals of Gerontology Series A: Biological Sciences and Medical Sciences. It was supported by an NIH/National Heart, Lung, and Blood Institute contract and training grant, the National Institute on Aging, the National Institute of Neurological Disorders and Stroke, and the American Heart Association.
New neurons created through exercise don’t cause you to forget old memories
Meanwhile, Texas A&M College of Medicine scientists have found in a study recently published in the Journal of Neuroscience that exercise causes more new neurons to be formed in a critical brain region — and contrary to an earlier study, these new neurons do not cause the individual to forget old memories.
Exercise is well known for its cognitive benefits, thought to occur because it causes neurogenesis, or the creation of new neurons, in the hippocampus, which is a key brain region for learning, memory and mood regulation. So it was a surprise in 2014 when a research study, published in the journal Science, found that exercise caused mice to forget what they’d already learned.
“It stunned the field of hippocampal neurogenesis,” said Ashok K. Shetty, PhD, a professor in the Texas A&M College of Medicine Department of Molecular and Cellular Medicine, associate director of the Institute for Regenerative Medicine, and research career scientist at the Central Texas Veterans Health Care System.
The animal models in the exercise group — in the previous study — showed far more neurogenesis than the control group, but these additional neurons seemed to erase memories that were formed before they started the exercise regimen. To test this, the researchers removed the extra neurons, and the mice suddenly were able to remember again.
Replicating the research with rats reversed the outcome
Shetty and his team decided to replicate this earlier research, using rats instead of mice. Rats are thought to be more like humans physiologically, with more-similar neuronal workings. They found that these animal models showed no such degradation in memories.
The researchers trained their animal models to complete a task over the course of four days, followed by several days of memory consolidation by performing the task over and over again. Then, half the trained animal models were put into cages with running wheels for several weeks, while the control group remained sedentary.
The rats who ran further over the course of that time had much greater neurogenesis in their hippocampus, and all rats who had access to a wheel (and therefore ran at least some), had greater neurogenesis than the sedentary group.
Importantly, despite differing levels of increased neurogenesis, both moderate runners and brisk runners (those who ran further than average) in Shetty’s study showed the same ability as the sedentary runners to recall the task they learned before they began to exercise.
This means even a large amount of running (akin to people who perform significant amount of exercise on a daily basis) doesn’t interfere with the recall of memory.
Why fidgeting can protect leg arteries and improve learning

(credit: Dave Clark/National Education Association)
But not everybody has that extra hour — or is motivated to exercise. For all of us couch/desk-bound folks, new research suggests a simple sitting exercise that may tide you over until you can go for a walk or run: fidgeting.
Ignore that teacher advice to “sit in your seat and don’t fidget!” — which the National Education Association says in this article actually improves learning — and suggests footrests using old tires to make giant rubber bands to facilitate fidgeting.
University of Missouri researchers have found that fidgeting while sitting can also protect the arteries in legs and potentially help prevent the arterial disease that may be brought on by binge TV watching or working at a computer.
In the study, the researchers compared the leg vascular function of 11 healthy young men and women before and after three hours of sitting. While sitting, the participants were asked to fidget one leg intermittently, tapping one foot for one minute and then resting it for four minutes, while the other leg remained still throughout. On average, the participants moved their feet 250 times per minute.
The researchers then measured the blood flow of the popliteal — an artery in the lower leg — and found that the fidgeting leg had a significant increase in blood flow, as expected, while the stationary leg experienced a reduction in blood flow.
Research has shown that increased blood flow and its associated shear stress — the friction of the flowing blood on the artery wall — is an important stimulus for vascular health.
While only one leg was exposed to fidgeting during the experiment, in a real-world scenario the researchers recommend tapping both legs to maximize the beneficial effects.
But not a substitute for walking and exercise
“Many of us sit for hours at a time, whether it’s binge watching our favorite TV show or working at a computer,” said Jaume Padilla, PhD, an assistant professor of nutrition and exercise physiology at MU and lead author of the study.
“We wanted to know whether a small amount of leg fidgeting could prevent a decline in leg vascular function caused by prolonged sitting. While we expected fidgeting to increase blood flow to the lower limbs, we were quite surprised to find this would be sufficient to prevent a decline in arterial function.”
But fidgeting is not a substitute for walking and exercise, which produce more overall cardiovascular benefits, the researchers caution.
“You should attempt to break up sitting time as much as possible by standing or walking,” Padilla said. “But if you’re stuck in a situation in which walking just isn’t an option, fidgeting can be a good alternative. Any movement is better than no movement.”
The study was recently published in the American Journal of Physiology Heart and Circulatory Physiology and was supported by the NIH and the Japan Society for the Promotion of Science.
Abstract of Physical Activity, Brain Volume, and Dementia Risk: The Framingham Study
Background: Several longitudinal studies found an inverse relationship between levels of physical activity and cognitive decline, dementia, and/or Alzheimer’s disease (AD), but results have been inconsistent. We followed an older, community-based cohort for over a decade to examine the association of physical activity with the risk of incident dementia and subclinical brain MRI markers of dementia.
Methods: The physical activity index (PAI) was assessed in the Framingham Study Original and Offspring cohorts, aged 60 years or older. We examined the association between PAI and risk of incident all-cause dementia and AD in participants of both cohorts who were cognitively intact and had available PAI (n = 3,714; 54% women; mean age = 70±7 years). We additionally examined the association between PAI and brain MRI in the Offspring cohort (n = 1,987).
Results: Over a decade of follow-up, 236 participants developed dementia (188 AD). Participants in the lowest quintile of PAI had an increased risk of incident dementia compared with those in higher quintiles (hazard ratio [HR] = 1.50, 95% confidence interval [CI] = 1.04–1.97, p = .028) in a multivariable-adjusted model. Secondary analysis revealed that this relation was limited to participants who were apolipoprotein (APO)E ε4 allele noncarriers (HR = 1.58, 95% CI = 1.08–2.32; p = .018) and strongest in participants aged 75 years or older. PAI was also linearly related to total brain and hippocampal volumes (β ± SE = 0.24±0.06; p < .01 and 0.004±0.001; p = .003, respectively).
Conclusion: Low physical activity is associated with a higher risk for dementia in older individuals, suggesting that a reduced risk of dementia and higher brain volumes may be additional health benefits of maintaining physical activity into old age.
Abstract of Voluntary Running Exercise-Mediated Enhanced Neurogenesis Does Not Obliterate Retrograde Spatial Memory
Running exercise (RE) improves cognition, formation of anterograde memories, and mood, alongside enhancing hippocampal neurogenesis. A previous investigation in a mouse model showed that RE-induced increased neurogenesis erases retrograde memory (Akers et al., 2014). However, it is unknown whether RE-induced forgetting is common to all species. We ascertained whether voluntary RE-induced enhanced neurogenesis interferes with the recall of spatial memory in rats. Young rats assigned to either sedentary (SED) or running exercise (RE) groups were first subjected to eight learning sessions in a water maze. A probe test (PT) conducted 24 h after the final training session confirmed that animals in either group had a similar ability for the recall of short-term memory. Following this, rats in the RE group were housed in larger cages fitted with running wheels, whereas rats in the SED group remained in standard cages. Animals in the RE group ran an average of 78 km in 4 weeks. A second PT performed 4 weeks after the first PT revealed comparable ability for memory recall between animals in the RE and SED groups, which was evidenced through multiple measures of memory retrieval function. The RE group displayed a 1.5- to 2.1-fold higher hippocampal neurogenesis than SED rats. Additionally, both moderate and brisk RE did not interfere with the recall of memory, although increasing amounts of RE proportionally enhanced neurogenesis. In conclusion, RE does not impair memory recall ability in a rat model despite substantially increasing neurogenesis.
SIGNIFICANCE STATEMENT Running exercise (RE) improves new memory formation along with an increased neurogenesis in the hippocampus. In view of a recent study showing that RE-mediated increased hippocampal neurogenesis promotes forgetfulness in a mouse model, we ascertained whether a similar adverse phenomenon exists in a rat model. Memory recall ability examined 4 weeks after learning confirmed that animals that had run a mean of 78 km and displayed a 1.5- to 2.1-fold increase in hippocampal neurogenesis demonstrated similar proficiency for memory recall as animals that had remained sedentary. Furthermore, both moderate and brisk RE did not interfere with memory recall, although increasing amounts of RE proportionally enhanced neurogenesis, implying that RE has no adverse effects on memory recall.
Abstract of Prolonged sitting-induced leg endothelial dysfunction is prevented by fidgeting
Prolonged sitting impairs endothelial function in the leg vasculature, and this impairment is thought to be largely mediated by a sustained reduction in blood flow-induced shear stress. Indeed, preventing the marked reduction of shear stress during sitting with local heating abolishes the impairment in popliteal artery endothelial function. Herein, we tested the hypothesis that sitting-induced reductions in shear stress and ensuing endothelial dysfunction would be prevented by periodic leg movement, or “fidgeting.” In 11 young, healthy subjects, bilateral measurements of popliteal artery flow-mediated dilation (FMD) were performed before and after a 3-h sitting period during which one leg was subjected to intermittent fidgeting (1 min on/4 min off) while the contralateral leg remained still throughout and served as an internal control. Fidgeting produced a pronounced increase in popliteal artery blood flow and shear rate (prefidgeting, 33.7 ± 2.6 s−1 to immediately postfidgeting, 222.7 ± 28.3 s−1; mean ± SE; P < 0.001) that tapered off during the following 60 s. Fidgeting did not alter popliteal artery blood flow and shear rate of the contralateral leg, which was subjected to a reduction in blood flow and shear rate throughout the sitting period (presit, 71.7 ± 8.0 s−1 to 3-h sit, 20.2 ± 2.9 s−1; P < 0.001). Popliteal artery FMD was impaired after 3 h of sitting in the control leg (presit, 4.5 ± 0.3% to postsit: 1.6 ± 1.1%; P = 0.039) but improved in the fidgeting leg (presit, 3.7 ± 0.6% to postsit, 6.6 ± 1.2%; P = 0.014). Collectively, the present study provides evidence that prolonged sitting-induced leg endothelial dysfunction is preventable with small amounts of leg movement while sitting, likely through the intermittent increases in vascular shear stress.