(credit: iStock)
Cognitive brain training improves executive function while aerobic activity improves memory, according to a new study by the Center for BrainHealth at The University of Texas at Dallas.
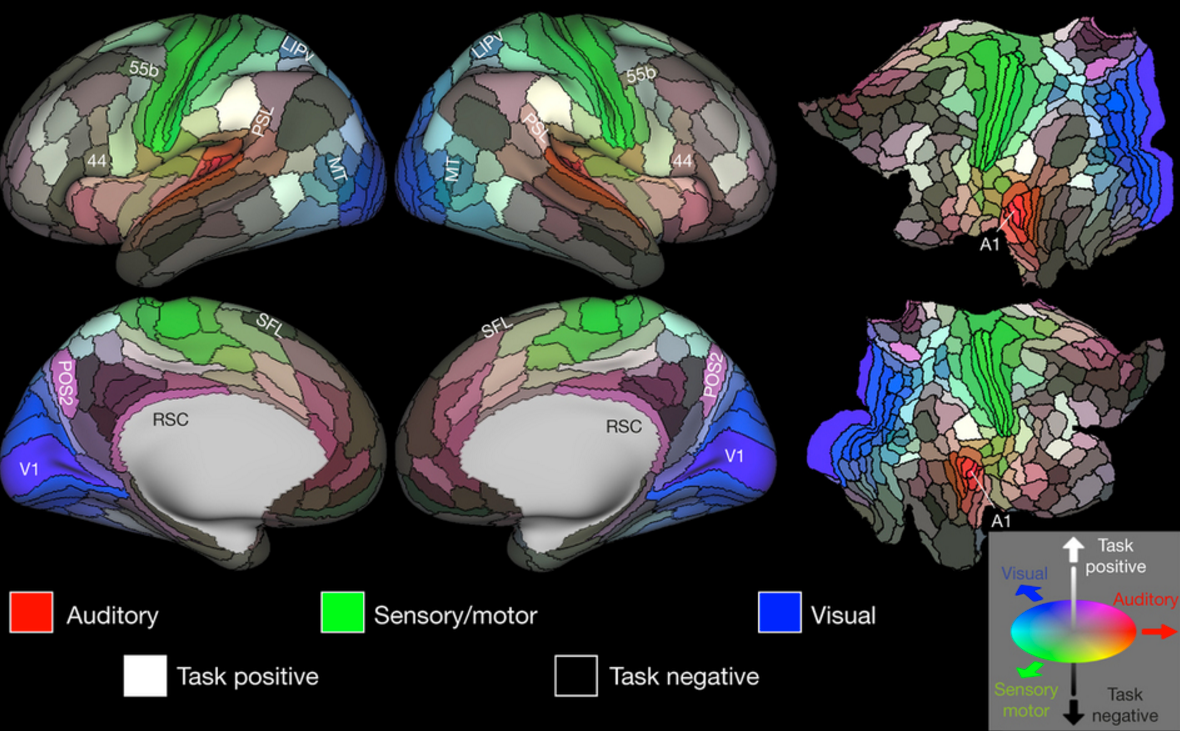
The study, published in an open-access paper in Frontiers in Human Neuroscience, compared cerebral blood flow and cerebrovascular reactivity data, obtained via MRI, for two groups of healthy sedentary adults ages 56–75 years. The members of both groups participated in training three hours per week over 12 weeks.
Cognitive training group
This group participated in cognitive training called Strategic Memory Advanced Reasoning Training (SMART), developed at the Center for BrainHealth. It focuses on three executive functions: strategic attention (prioritizing brain resources); integrative reasoning (synthesizing information at a deeper level); and innovation (encouraging fluid thinking, diverse perspective-taking, and problem solving).
The group demonstrated positive changes in executive brain function and a 7.9 percent increase in global brain flow.
“We can lose 1–2 percent in global brain blood flow every decade, starting in our 20s. To see almost an 8 percent increase in brain blood flow may be seen as regaining decades of brain health, since blood flow is linked to neural health,” said Sandra Bond Chapman, PhD, study lead author, founder and chief director of the Center for BrainHealth, and Dee Wyly Distinguished University Professor.
“We believe the reasoning training triggered neural plasticity by engaging the brain networks involved in staying focused on a goal, such as writing a brief business proposal, while continuously adapting to new information, such as feedback from a collaborator,” Chapman said.
Aerobic exercise group
The aerobic exercise group completed three, 60-minute sessions per week that included five minutes of warmup and cool down with 50 minutes of either walking on a treadmill or cycling on a stationary bike while maintaining 50–75 percent of maximum heart rate. It was designed to meet health guidelines for adults.
The group showed increases in immediate and delayed memory performance, with higher cerebral blood flow in the bilateral hippocampi, an area underlying memory function and particularly vulnerable to aging and dementia. But the group did not show significant global blood flow gains.
This work was supported by a grant from the National Institutes of Health and by grants from the Lyda Hill Foundation, T. Boone Pickens Foundation, and the Dee Wyly Distinguished University Endowment.
Abstract of Distinct Brain and Behavioral Benefits from Cognitive vs. Physical Training: A Randomized Trial in Aging Adults
Insidious declines in normal aging are well-established. Emerging evidence suggests that non-pharmacological interventions, specifically cognitive and physical training, may counter diminishing age-related cognitive and brain functions. This randomized trial compared effects of two training protocols: cognitive training (CT) vs. physical training (PT) on cognition and brain function in adults 56–75 years. Sedentary participants (N = 36) were randomized to either CT or PT group for 3 h/week over 12 weeks. They were assessed at baseline-, mid-, and post-training using neurocognitive, MRI, and physiological measures. The CT group improved on executive function whereas PT group’s memory was enhanced. Uniquely deploying cerebral blood flow (CBF) and cerebral vascular reactivity (CVR) MRI, the CT cohort showed increased CBF within the prefrontal and middle/posterior cingulate cortex (PCC) without change to CVR compared to PT group. Improvements in complex abstraction were positively associated with increased resting CBF in dorsal anterior cingulate cortex (dACC). Exercisers with higher CBF in hippocampi bilaterally showed better immediate memory. The preliminary evidence indicates that increased cognitive and physical activity improves brain health in distinct ways. Reasoning training enhanced frontal networks shown to be integral to top-down cognitive control and brain resilience. Evidence of increased resting CBF without changes to CVR implicates increased neural health rather than improved vascular response. Exercise did not improve cerebrovascular response, although CBF increased in hippocampi of those with memory gains. Distinct benefits incentivize testing effectiveness of combined protocols to strengthen brain health.