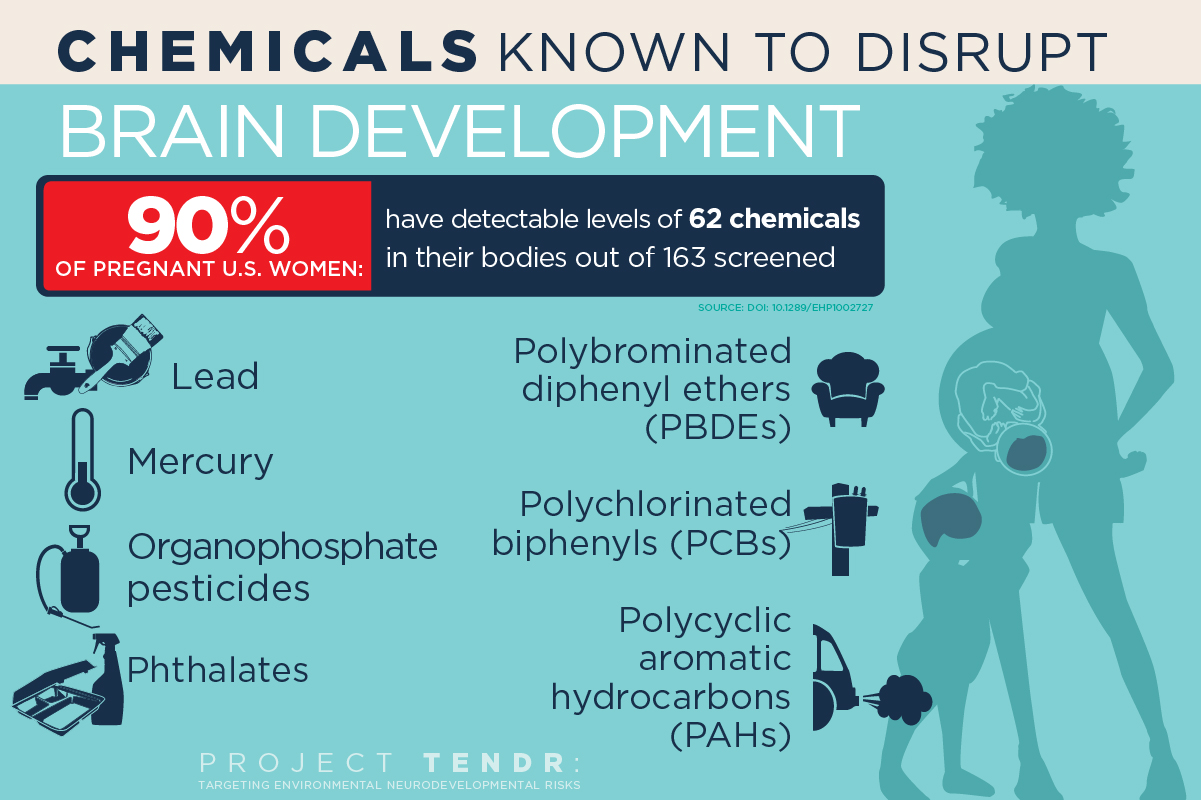
In a new open-access report in the NIH journal Environmental Health Perspectives, 47 scientists, health practitioners, and children’s health advocates have made a consensus statement in “Project TENDR: Targeting Environmental Neuro-Developmental Risks“ — endorsed by nine medical organizations — and issued a call to action for renewed attention to the growing evidence that many common and widely available chemicals endanger neurodevelopment in fetuses and children of all ages.
The list includes chemicals used extensively in consumer products and that have become widespread in the environment. Of most concern are lead and mercury; organophosphate pesticides used in agriculture and home gardens; phthalates, which are used in pharmaceuticals, plastics and personal care products; flame retardants known as polybrominated diphenyl ethers; and air pollutants produced by the combustion of wood and fossil fuels, said University of Illinois Comparative Biosciences professor Susan Schantz, one of dozens of individual signatories to the consensus statement.
The list provides “prime examples of toxic chemicals that can contribute to learning, behavioral, or intellectual impairment, as well as specific neurodevelopmental disorders such as ADHD or autism spectrum disorder,” according to the report.
Polychlorinated biphenyls
Polychlorinated biphenyls, once used as coolants and lubricants in transformers and other electrical equipment, also are of concern. PCBs were banned in the U.S. in 1977, but can persist in the environment for decades, she said.
“These chemicals are pervasive, not only in air and water, but in everyday consumer products that we use on our bodies and in our homes,” Schantz said. “Reducing exposures to toxic chemicals can be done, and is urgently needed to protect today’s and tomorrow’s children.”
“The human brain develops over a very long period of time, starting in gestation and continuing during childhood and even into early adulthood,” Schantz said. “But the biggest amount of growth occurs during prenatal development. The neurons are forming and migrating and maturing and differentiating. And if you disrupt this process, you’re likely to have permanent effects.”
Hormonal disrupters
Some of the chemicals of concern, such as phthalates and PBDEs, are known to interfere with normal hormone activity. For example, most pregnant women in the U.S. will test positive for exposure to phthalates and PBDEs, both of which disrupt thyroid hormone function.
“Thyroid hormone is involved in almost every aspect of brain development, from formation of the neurons to cell division, to the proper migration of cells and myelination of the axons after the cells are differentiated,” said Schantz. “It regulates many of the genes involved in nervous system development.”
Schantz and her colleagues at Illinois are studying infants and their mothers to determine whether prenatal exposure to phthalates and other endocrine disruptors leads to changes in the brain or behavior. This research, along with parallel studies in older children and animals, is a primary focus of the Children’s Environmental Health Research Center at Illinois, which Schantz directs.
Phthalates also interfere with steroid hormone activity. Studies link exposure to certain phthalates with attention deficits, lower IQ and conduct disorders in children. “Phthalates are everywhere; they’re in all kinds of different products. We’re exposed to them every day,” Schantz said.
The report criticizes current regulatory lapses that allow chemicals to be introduced into people’s lives with little or no review of their effects on fetal and child health. “For most chemicals, we have no idea what they’re doing to children’s neurodevelopment,” Schantz said. “They just haven’t been studied.
“And if it looks like something is a risk, we feel policymakers should be willing to make a decision that this or that chemical could be a bad actor and we need to stop its production or limit its use,” she said. “We shouldn’t have to wait 10 or 15 years — allowing countless children to be exposed to it in the meantime — until we’re positive it’s a bad actor.”
Project TENDR has a website with information about each of the chemicals of concern. The National Institute of Environmental Health Sciences at the National Institutes of Health and the U.S. Environmental Protection Agency fund the Children’s Environmental Health Research Center at the University of Illinois.
Project TENDR is an alliance of 48 of the nation’s top scientists, health professionals and health advocates. It was launched by Maureen Swanson of the Learning Disabilities Association of America and Irva Hertz-Picciotto of UC Davis, who brought together participants across many disciplines and sectors, including epidemiology, toxicology, exposure science, pediatrics, obstetrics and gynecology, nursing, public health, and federal and state chemical policy. Medical and scientific societies that have signed on in support include American Congress of Obstetricians and Gynecologists, American Nurses Association, Endocrine Society, National Association of Pediatric Nurse Practitioners, National Medical Association, National Hispanic Medical Association, Alliance of Nurses for Healthy Environments, Physicians for Social Responsibility and the National Council of Asian Pacific Island Physicians. TENDR’s long-term mission is to lower the incidence of neurodevelopmental disorders by reducing exposure levels to chemicals and pollutants that can contribute to these conditions, especially during fetal development and early childhood.
Steve Drake, Beckman Institute for Advanced Science and Technology | U. of I. biosciences professor Susan Schantz directs the Children’s Environmental Health Research Center at the University of Illinois, which is studying whether, and how, exposure to phthalates disrupts child brain development. Phthalates are used in some cosmetics, food packaging and products with fragrances.
Food Safety | From DDT to Glyphosate: Rachel Carson, We Need You Again
Abstract of Project TENDR: Targeting Environmental Neuro-Developmental Risks. The TENDR Consensus Statement
SUMMARY: Children in America today are at an unacceptably high risk of developing neurodevelopmental disorders that affect the brain and nervous system including autism, attention deficit hyperactivity disorder, intellectual disabilities, and other learning and behavioral disabilities. These are complex disorders with multiple causes—genetic, social, and environmental. The contribution of toxic chemicals to these disorders can be prevented. APPROACH: Leading scientific and medical experts, along with children’s health advocates, came together in 2015 under the auspices of Project TENDR: Targeting Environmental Neuro-Developmental Risks to issue a call to action to reduce widespread exposures to chemicals that interfere with fetal and children’s brain development. Based on the available scientific evidence, the TENDR authors have identified prime examples of toxic chemicals and pollutants that increase children’s risks for neurodevelopmental disorders. These include chemicals that are used extensively in consumer products and that have become widespread in the environment. Some are chemicals to which children and pregnant women are regularly exposed, and they are detected in the bodies of virtually all Americans in national surveys conducted by the U.S. Centers for Disease Control and Prevention. The vast majority of chemicals in industrial and consumer products undergo almost no testing for developmental neurotoxicity or other health effects. CONCLUSION: Based on these findings, we assert that the current system in the United States for evaluating scientific evidence and making health-based decisions about environmental chemicals is fundamentally broken. To help reduce the unacceptably high prevalence of neurodevelopmental disorders in our children, we must eliminate or significantly reduce exposures to chemicals that contribute to these conditions. We must adopt a new framework for assessing chemicals that have the potential to disrupt brain development and prevent the use of those that may pose a risk. This consensus statement lays the foundation for developing recommendations to monitor, assess, and reduce exposures to neurotoxic chemicals. These measures are urgently needed if we are to protect healthy brain development so that current and future generations can reach their fullest potential.