This is an artistic representation of the take home messages in Lunghi and Sale: “A cycling lane for brain rewiring,” which is that physical activity (such as cycling) is associated with increased brain plasticity. (credit: Dafne Lunghi Art)
Exercise may enhance plasticity of the adult brain — the ability of our neurons to change with experience — which is essential for learning, memory, and brain repair, Italian researchers report in an open-access paper in the Cell Press journal Current Biology.
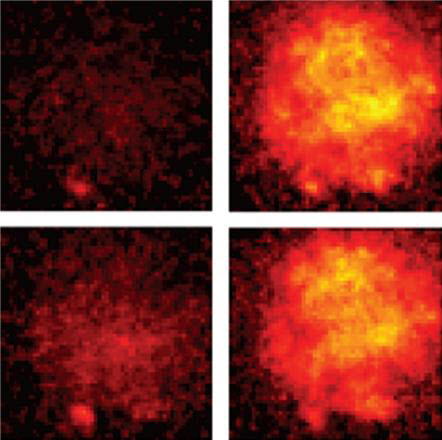
Their research, which focused on the the visual cortex, may offer hope for people with traumatic brain injury or eye conditions such as amblyopia, the researchers suggest. “We provide the first demonstration that moderate levels of physical activity enhance neuroplasticity in the visual cortex of adult humans,” says Claudia Lunghi of the University of Pisa in Italy.
Brain plasticity is generally thought to decline with age, especially in the sensory region of the brain (such as vision). But previous studies by research colleague Alessandro Sale of the National Research Council’s Neuroscience Institute showed that animals performing physical activity — for example rats running on a wheel — showed elevated levels of plasticity in the visual cortex and had improved recovery from amblyopia compared to more sedentary animals.
Binocular rivalry test
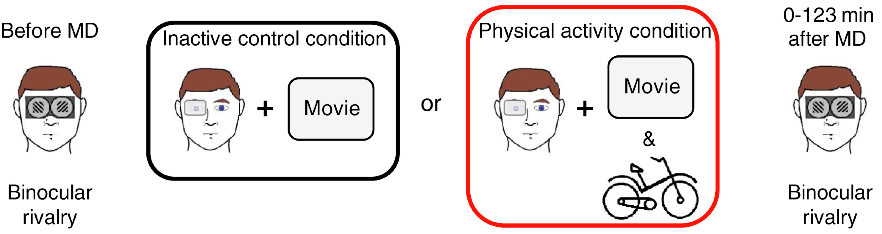
Binocular rivalry before and after “monocular deprivation” (reduced vision due to a patch) for inactive and active groups (credit: Claudia Lunghi and Alessandro Sale/Current Biology)
To find out whether the same might hold true for people, the researchers used a simple test of binocular rivalry. When people have one eye patched for a short period of time, the closed eye becomes stronger as the visual brain attempts to compensate for the lack of visual input. This recovered strength (after the eye patch is removed) is a measure of the brain’s visual plasticity.
In the new study, Lunghi and Sale put 20 adults through this test twice. In one test, participants with the dominant eye patched with a translucent material watched a movie while relaxing in a chair. In the other test, participants with one eye patched also watched a movie, but while exercising on a stationary bike for ten-minute intervals during the movie.
Exercise enhances brain plasticity (at least for vision)
Result: brain plasticity in the patched eye was enhanced by the exercise. After physical activity, the patched eye was strengthened more quickly (indicating increased levels of brain plasticity) than with the couch potatoes.
While further study is needed, the researchers think this stronger vision may have resulted from a decrease in an inhibitory neurotransmitter called GABA caused by exercise, allowing the brain to become more responsive.
The findings suggest that exercise may play an important role in brain health and recovery. This could be especially good news for people with amblyopia (called “lazy eye” because the brain “turns off” the visual processing of the weak eye to prevent double vision) — generally considered to be untreatable in adults.
Lunghi and Sale say they now plan to investigate the effects of moderate levels of physical exercise on visual function in amblyopic adult patients and to look deeper into the underlying neural mechanisms.
Time for a walk or bike ride?
UPDATE Dec. 10, 2o15: title wording changed from “smarter” to “learn better.”
Abstract of A cycling lane for brain rewiring
Brain plasticity, defined as the capability of cerebral neurons to change in response to experience, is fundamental for behavioral adaptability, learning, memory, functional development, and neural repair. The visual cortex is a widely used model for studying neuroplasticity and the underlying mechanisms. Plasticity is maximal in early development, within the so-called critical period, while its levels abruptly decline in adulthood. Recent studies, however, have revealed a significant residual plastic potential of the adult visual cortex by showing that, in adult humans, short-term monocular deprivation alters ocular dominance by homeostatically boosting responses to the deprived eye. In animal models, a reopening of critical period plasticity in the adult primary visual cortex has been obtained by a variety of environmental manipulations, such as dark exposure, or environmental enrichment, together with its critical component of enhanced physical exercise. Among these non-invasive procedures, physical exercise emerges as particularly interesting for its potential of application to clinics, though there has been a lack of experimental evidence available that physical exercise actually promotes visual plasticity in humans. Here we report that short-term homeostatic plasticity of the adult human visual cortex induced by transient monocular deprivation is potently boosted by moderate levels of voluntary physical activity. These findings could have a bearing in orienting future research in the field of physical activity application to clinical research.