Rayleigh scattering causes the reddening of the sun at sunset — an example of how longer wavelengths (yellow and red compared to blue in blue sky) penetrate matter (dust at sunset) better. (credit: Wikipedia/CC)
Researchers at The City College of New York (CCNY) have determined the optimal wavelengths for bioimaging of the brain at longer near-infrared wavelengths, which permit deeper imaging.
Near-infrared (NIR) radiation has been used for one- and two-photon fluorescence imaging at near-infrared wavelengths of 650–950 nm (nanometers) for deep brain imaging, but it is limited in penetration depth. (The CCNY researchers dubbed this Window I, also known as the therapeutic window.)
Longer infrared wavelengths penetrate deeper but are limited by Rayleigh and Mie scattering, which blur images, and absorption, which reduces the number of available photons (brightness). These limitations are based on the lack of suitable CMOS semiconductor imaging detectors or femtosecond laser sources.
The new CCNY study, led by biomedical engineer Lingyan Shi, studied three new optical windows in the near-infrared (NIR) region, in addition to Window I, for high-resolution deep brain imaging.
Their study built on a prior CCNY study* in 2014 using detectors based on indium gallium arsenide (GaAs) or indium antimonide-(InSb) and a femtosecond excitation source of IMRA fiber laser** to image rat brain tissue in window II (1,100– 1,350 nm), window III (1,600–1,870 nm), and window IV (1600 nm to 1870 nm, centered at 2,200 nm).
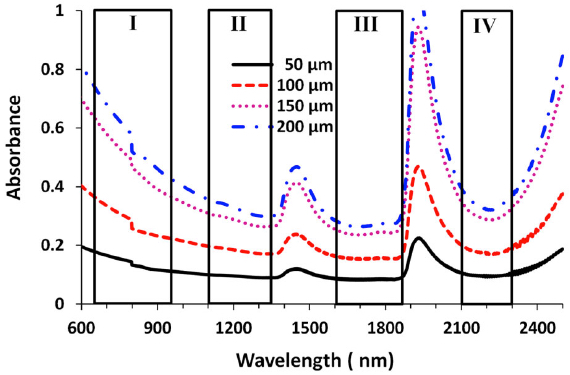
Absorbance for four windows at four brain-tissue thicknesses (credit: Lingyan Shi et al./J. Biophotonics)
The new CCNY research investigated the optimal wavelength band and optical properties of brain tissue with NIR, including the total attenuation coefficient (μt), absorption coefficient (μa), reduced scattering coefficient (μ0 s), and the scattering anisotropy coefficient (g) in these optical windows. The purpose of the study was to determine an optimal optical window in NIR in the 650 nm to 2500 nm range to reduce scattering, achieve optimal absorption, and reduce noise for deep-brain tissue imaging.
Golden Window: optimal wavelength range

Peak transmittance T (%) measured with each optical window for brain tissues with four thicknesses. Window III had the highest transmittance percentage for each of the thicknesses, followed by windows II and IV. (credit: Lingyan Shi et al./J. Biophotonics)
The researchers found that the “Golden Window” (1600 nm to 1870 nm) is an optimal wavelength range for light penetration in brain tissue, followed by Windows II and IV.
“This is a first for brain imaging and proved theoretically and experimentally that deep imaging of the brain is possible using light at longer wavelengths. It demonstrates these windows’ potential for deeper brain tissue imaging due to the reduction of scattering that causes blurring,” said Shi, a research associate in City College’s Institute for Ultrafast Spectroscopy and Lasers, and the biology department.
Published by the Journal of Biophotonics, her study sheds light on the development of the next generation of microscopy imaging technique, in which the “Golden Window” may be utilized for high-resolution deeper brain imaging. The next step in the research is in vivo imaging in mice using Golden Window wavelength light.
Shi’s team included Distinguished Professor of Physics Robert R. Alfano and Adrian Rodriguez-Contreras, an assistant professor of biology. Shi earned a Ph.D. in biomedical engineering from CCNY’s Grove School of Engineering in 2014.
* L. A. Sordillo, Y. Pu, S. Pratavieira, Y. Budansky, and R. R. Alfano, J. Biomed. Opt. 19, 056004 (2014) [link].
** Excitation wavelength 1680 nm, power > 200 mW, pulse width 100 fs, and 50 MHz repetition rate.
Abstract of Transmission in near-infrared optical windows for deep brain imaging
Near-infrared (NIR) radiation has been employed using one- and two-photon excitation of fluorescence imaging at wavelengths 650–950 nm (optical window I) for deep brain imaging; however, longer wavelengths in NIR have been overlooked due to a lack of suitable NIR-low band gap semiconductor imaging detectors and/or femtosecond laser sources. This research introduces three new optical windows in NIR and demonstrates their potential for deep brain tissue imaging. The transmittances are measured in rat brain tissue in the second (II, 1,100–1,350 nm), third (III, 1,600–1,870 nm), and fourth (IV, centered at 2,200 nm) NIR optical tissue windows. The relationship between transmission and tissue thickness is measured and compared with the theory. Due to a reduction in scattering and minimal absorption, window III is shown to be the best for deep brain imaging, and windows II and IV show similar but better potential for deep imaging than window I.