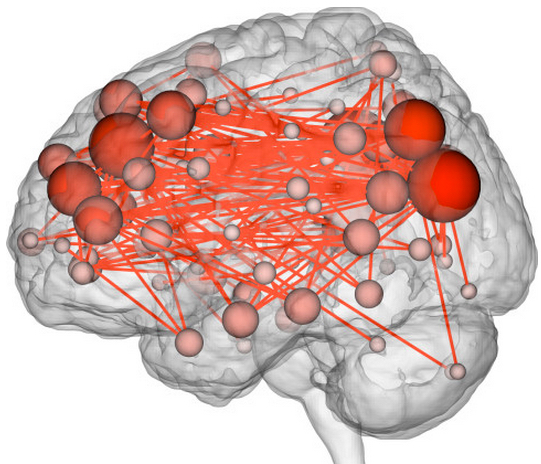
Drugs used to treat a variety of central nervous system diseases may be administered through the nose and diffused through an implanted mucosal graft (left, in red) to gain access to the brain. Under normal circumstances, there are multiple layers within the nose that block the access of pharmaceutical agents from getting to the brain, including bone and the dura/arachnoid membrane, which represents part of the blood-brain barrier (top right). After endoscopic skull base surgery (bottom right), all of these layers are removed and replaced with a nasal mucosal graft, which is 1,000 times more porous than the native blood-brain barrier. So these grafts may be used to deliver very large drugs, including proteins, which would otherwise be blocked by the blood-brain barrier. (credit: Garyfallia Pagonis and Benjamin S. Bleier, M.D.)
Researchers at Massachusetts Eye and Ear/Harvard Medical School and Boston University have developed a new technique to deliver drugs across the blood-brain barrier and have successfully tested it in a Parkinson’s mouse model (a line of mice that has been genetically modified to express the symptoms and pathological features of Parkinson’s to various extents).
Their findings, published in the journal Neurosurgery, lend hope to patients with neurological conditions that are difficult to treat due to a barrier mechanism that prevents approximately 98 percent of drugs from reaching the brain and central nervous system.
“Although we are currently looking at neurodegenerative disease, there is potential for the technology to be expanded to psychiatric diseases, chronic pain, seizure disorders, and many other conditions affecting the brain and nervous system down the road,” said senior author Benjamin S. Bleier, M.D., of the department of otolaryngology at Mass. Eye and Ear/Harvard Medical School.
The nasal mucosal grafting solution
Researchers delivered glial derived neurotrophic factor (GDNF), a therapeutic protein in testing for treating Parkinson’s disease, to the brains of mice. They showed that their delivery method was equivalent to direct injection of GDNF, which has been shown to delay and even reverse disease progression of Parkinson’s disease in pre-clinical models.
Once they have finished the treatment, they use adjacent nasal lining to rebuild the hole in a permanent and safe way. Nasal mucosal grafting is a technique regularly used in the ENT (ear, nose, and throat) field to reconstruct the barrier around the brain after surgery to the skull base. ENT surgeons commonly use endoscopic approaches to remove brain tumors through the nose by making a window through the blood-brain barrier to access the brain.
The safety and efficacy of these methods have been well established through long-term clinical outcomes studies in the field, with the nasal lining protecting the brain from infection just as the blood brain barrier has done.
By functionally replacing a section of the blood-brain barrier with nasal mucosa, which is more than 1,000 times more permeable than the native barrier, surgeons could create a “screen door” to allow for drug delivery to the brain and central nervous system.
The technique has the potential to benefit a large population of patients with neurodegenerative disorders, where there is still a specific unmet need for blood-brain-penetrating therapeutic delivery strategies.
The study was funded by The Michael J. Fox Foundation for Parkinson’s Research (MJFF).
Abstract of Heterotopic Mucosal Grafting Enables the Delivery of Therapeutic Neuropeptides Across the Blood Brain Barrier
BACKGROUND: The blood-brain barrier represents a fundamental limitation in treating neurological disease because it prevents all neuropeptides from reaching the central nervous system (CNS). Currently, there is no efficient method to permanently bypass the blood-brain barrier.
OBJECTIVE: To test the feasibility of using nasal mucosal graft reconstruction of arachnoid defects to deliver glial-derived neurotrophic factor (GDNF) for the treatment of Parkinson disease in a mouse model.
METHODS: The Institutional Animal Care and Use Committee approved this study in an established murine 6-hydroxydopamine Parkinson disease model. A parietal craniotomy and arachnoid defect was repaired with a heterotopic donor mucosal graft. The therapeutic efficacy of GDNF (2 [mu]g/mL) delivered through the mucosal graft was compared with direct intrastriatal GDNF injection (2 [mu]g/mL) and saline control through the use of 2 behavioral assays (rotarod and apomorphine rotation). An immunohistological analysis was further used to compare the relative preservation of substantia nigra cell bodies between treatment groups.
RESULTS: Transmucosal GDNF was equivalent to direct intrastriatal injection at preserving motor function at week 7 in both the rotarod and apomorphine rotation behavioral assays. Similarly, both transmucosal and intrastriatal GDNF demonstrated an equivalent ratio of preserved substantia nigra cell bodies (0.79 +/- 0.14 and 0.78 +/- 0.09, respectively, P = NS) compared with the contralateral control side, and both were significantly greater than saline control (0.53 +/- 0.21; P = .01 and P = .03, respectively).
CONCLUSION: Transmucosal delivery of GDNF is equivalent to direct intrastriatal injection at ameliorating the behavioral and immunohistological features of Parkinson disease in a murine model. Mucosal grafting of arachnoid defects is a technique commonly used for endoscopic skull base reconstruction and may represent a novel method to permanently bypass the blood-brain barrier.