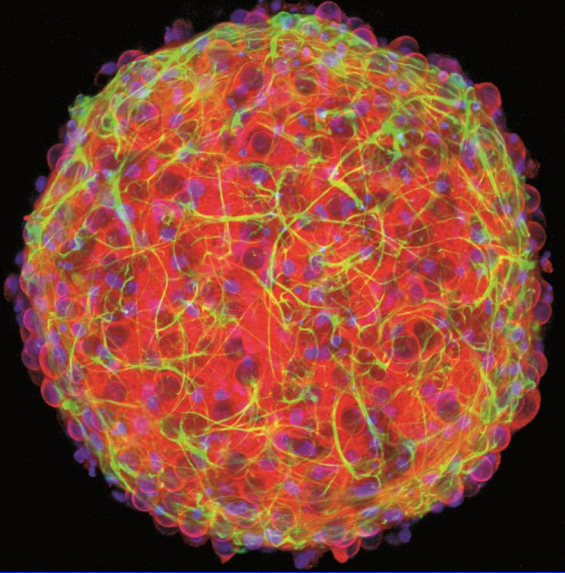
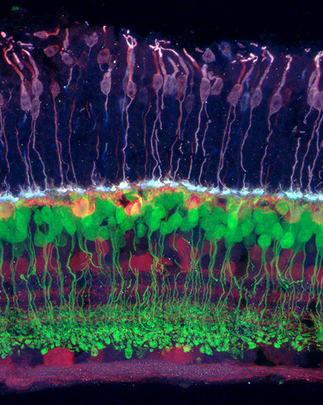
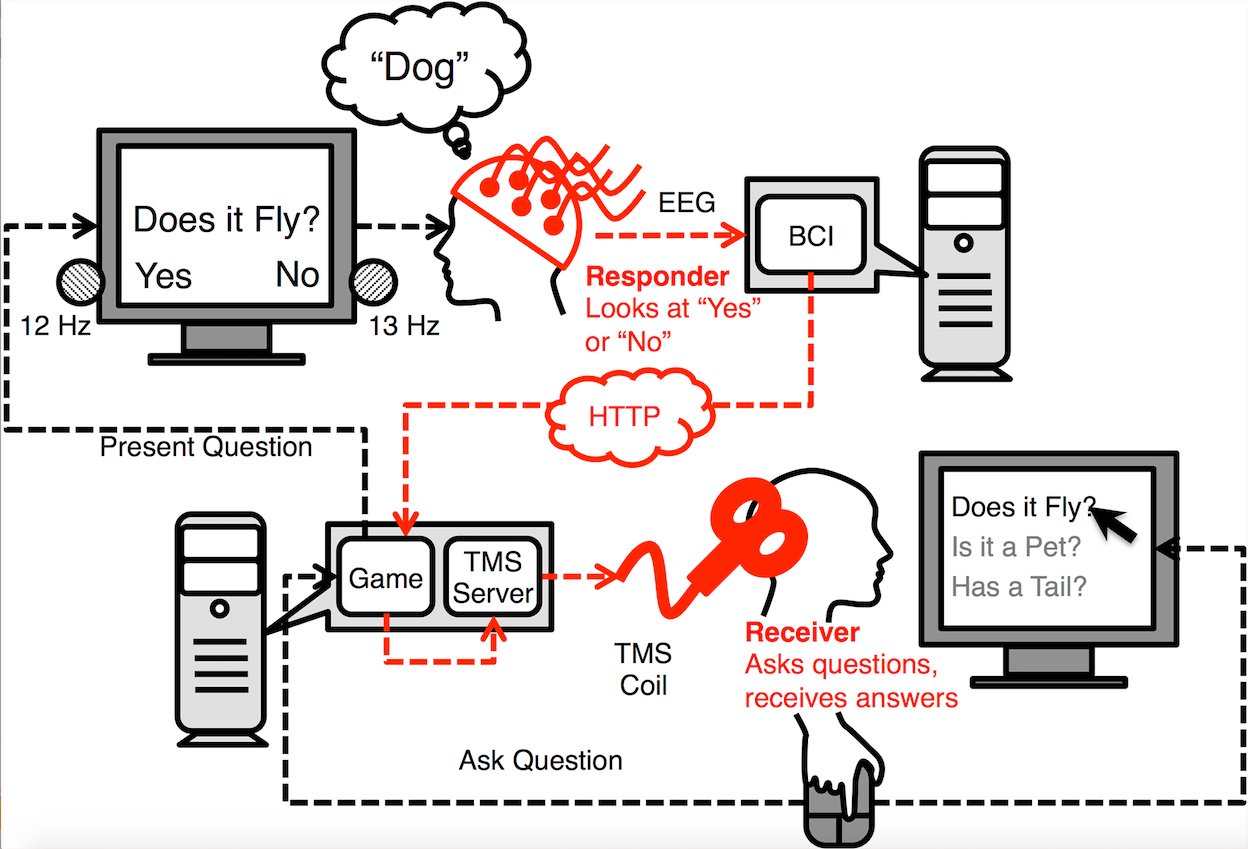
Salk scientists developed a new technique to grow aged brain cells from patients’ skin. Fibroblasts (cells in connective tissue) from elderly human donors are directly converted into induced neurons, as shown here. (credit: Salk Institute)
Scientists have developed a first-ever technique for using skin samples from older patients to create brain cells — without first rolling back the youthfulness clock in the cells. The new technique, which yields cells resembling those found in older people’s brains, will be a boon to scientists studying age-related diseases like Alzheimer’s and Parkinson’s.
“This lets us keep age-related signatures in the cells so that we can more easily study the effects of aging on the brain,” says Rusty Gage, a professor in the Salk Institute’s Laboratory of Genetics and senior author of the paper, published yesterday (October 8, 2015) in Cell Stem Cell.
“By using this powerful approach, we can begin to answer many questions about the physiology and molecular machinery of human nerve cells — not just around healthy aging but pathological aging as well,” says Martin Hetzer, a Salk professor also involved in the work.
Over the past few years, researchers have increasingly turned to human stem cells (instead of animals) to study various diseases in humans. For example, scientists can take patients’ skin cells and turn them into induced pluripotent stem cells, which have the ability to become any cell in the body. From there, researchers can prompt the stem cells to turn into brain cells for further study. But this process — even when taking skin cells from an older human — doesn’t guarantee stem cells with “older” properties.
Epigenetic signatures in older cells —patterns of chemical marks on DNA that dictate what genes are expressed when — were reset to match younger signatures in the process. This made studying the aging of the human brain difficult, since researchers couldn’t create “old” brain cells with the approach.
Induced neurons
The researchers decided to try another approach, turning to an even newer technique that lets them directly convert skin cells to neurons, creating what’s called an induced neuron. “A few years ago, researchers showed that it’s possible to do this, completely bypassing the stem cell precursor state,” says Jerome Mertens, a postdoctoral research fellow and first author of the new paper.
The scientists collected skin cells from 19 people, aged from birth to 89, and prompted them to turn into brain cells using both the induced pluripotent stem cell technique and the direct conversion approach. Then, they compared the patterns of gene expression in the resulting neurons with cells taken from autopsied brains.
When the induced pluripotent stem cell method was used, as expected, the patterns in the neurons were indistinguishable between young and old derived samples. But brain cells that had been created using the direct conversion technique had different patterns of gene expression depending on whether they were created from young donors or older adults.
“The neurons we derived showed differences depending on donor age,” says Mertens. “And they actually show changes in gene expression that have been previously implicated in brain aging.” For instance, levels of a nuclear pore protein called RanBP17 — whose decline is linked to nuclear transport defects that play a role in neurodegenerative diseases — were lower in the neurons derived from older patients.
Now that the direct conversion of skin cells to neurons has been shown to retain these signatures of age, Gage expects the technique to become a valuable tool for studying aging. And, while the current work only tested its effectiveness in creating brain cells, he suspects a similar method will let researchers create aged heart and liver cells as well.
Scientists at Friederich-Alexander University Erlangen-Nuremberg and Tsinghau University were also involved in the study, which was supported by grants from the G. Harold & Leila Y. Mathers Charitable Foundation, the JPB Foundation, the Leona M. and Harry B. Helmsley Charitable Trust, Annette Merle-Smith, CIRM, the German Federal Ministry of Education and Research, and the Glenn Foundation for Medical Research.
Abstract of Human induced neurons retain aging transcriptome signatures that identify compromised nucleocytoplasmic compartmentalization during aging
Aging is a major risk factor for many human diseases, and in vitro generation of human neurons is an attractive approach for modeling aging-related brain disorders. However, modeling aging in differentiated human neurons has proved challenging. We generated neurons from human donors across a broad range of ages, either by iPSC-based reprogramming and differentiation or by direct conversion into induced neurons (iNs). While iPSCs and derived neurons did not retain aging-associated gene signatures, iNs displayed age-specific transcriptional profiles and revealed age-associated decreases in the nuclear transport receptor RanBP17. We detected an age-dependent loss of nucleocytoplasmic compartmentalization (NCC) in donor fibroblasts and corresponding iNs, and found that reduced RanBP17 impaired NCC in young cells while iPSC rejuvenation restored NCC in aged cells. These results show that iNs retain important aging-related signatures, thus allowing modeling of the aging process in vitro, and identify impaired NCC as an important factor in human aging.