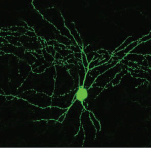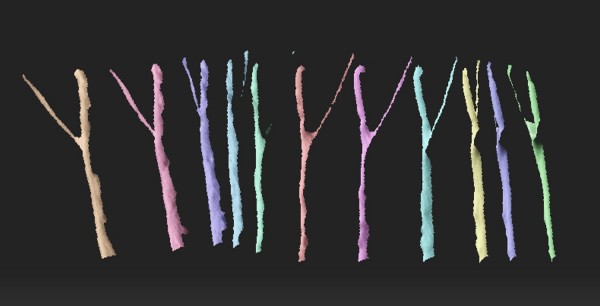
3-D scans of a nerve from different angles are used to create a custom regeneration guide for complex nerves (credit: University of Minnesota)
A national team of researchers used a combination of 3-D imaging and 3-D printing techniques to create a custom silicone guide implanted with biochemical cues to help nerve regeneration after an injury.
Nerve regeneration is a complex process, which is why regrowth of nerves after injury or disease is very rare and often permanent, according to the Mayo Clinic.
As a test, the researchers used a 3-D scanner to reverse-engineer the structure of a rat’s sciatic nerve. They then used a specialized, custom-built 3-D printer to print a regeneration guide containing 3D-printed chemical cues to promote both motor and sensory nerve regeneration within the same structure. The guide was then implanted into the rat by surgically grafting it to the cut ends of the nerve. Within about 10 to 12 weeks, the rat’s ability to walk again was improved.

A 3D-printed complex nerve-regeneration pathway implanted in a rat helped to improve walking in 10 to 12 weeks after implantation (credit: University of Minnesota)
“Someday we hope that we could have a 3D scanner and printer right at the hospital to create custom nerve guides right on site to restore nerve function,” said University of Minnesota mechanical engineering professor Michael McAlpine, the study’s lead researcher.
Conventional nerve guidance channels are typically fabricated around cylindrical substrates, so the resulting guidance devices are limited to linear structures. This is is the first time a study has shown the creation of a custom guide for regrowth of a complex nerve like the Y-shaped sciatic nerve, which has both sensory and motor branches.
“The exciting next step would be to implant these guides in humans rather than rats,” McAlpine said. For cases where a patient’s nerve is unavailable for scanning, McAlpine said there could someday be a “library” of scanned nerves from other people or cadavers that hospitals could use to create closely matched 3D-printed guides for patients.
The study by researchers from the University of Minnesota, Virginia Tech, University of Maryland, Princeton University, and Johns Hopkins University was published Thursday (Sept. 17) in the journal Advanced Functional Materials.
UMN College of Science and Engineering | 3D printing of a nerve regeneration guide [no audio]
Abstract of 3D Printed Anatomical Nerve Regeneration Pathways
A 3D printing methodology for the design, optimization, and fabrication of a custom nerve repair technology for the regeneration of complex peripheral nerve injuries containing bifurcating sensory and motor nerve pathways is introduced. The custom scaffolds are deterministically fabricated via a microextrusion printing principle using 3D models, which are reverse engineered from patient anatomies by 3D scanning. The bifurcating pathways are augmented with 3D printed biomimetic physical cues (microgrooves) and path-specific biochemical cues (spatially controlled multicomponent gradients). In vitro studies reveal that 3D printed physical and biochemical cues provide axonal guidance and chemotractant/chemokinetic functionality. In vivo studies examining the regeneration of bifurcated injuries across a 10 mm complex nerve gap in rats showed that the 3D printed scaffolds achieved successful regeneration of complex nerve injuries, resulting in enhanced functional return of the regenerated nerve. This approach suggests the potential of 3D printing toward advancing tissue regeneration in terms of: (1) the customization of scaffold geometries to match inherent tissue anatomies; (2) the integration of biomanufacturing approaches with computational modeling for design, analysis, and optimization; and (3) the enhancement of device properties with spatially controlled physical and biochemical functionalities, all enabled by the same 3D printing process.