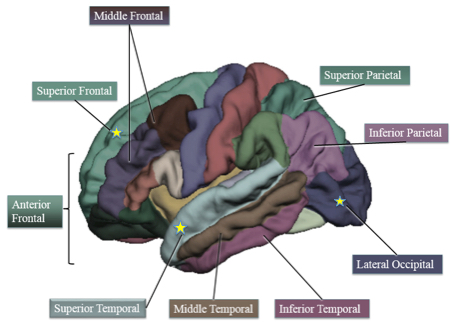
Cortical thickness regions of interest. Starred regions are areas in which higher-fit children showed decreased cortical thickness compared to lower-fit children. (credit: Laura Chaddock-Heyman et al./PLOS ONE)
A new study reveals that 9- and 10-year-old children who are aerobically fit tend to have significantly thinner gray matter than their “lower-fit” peers. Thinning of the outermost layer of brain cells in the cerebrum is associated with better mathematics performance, researchers report in an open-access paper in the journal PLOS ONE.
The study suggests, but does not prove, that cardiorespiratory fitness contributes to gray matter thinning — a normal process of child brain development. The study also offers the first evidence that fitness enhances math skills by aiding the development of brain structures that contribute to mathematics achievement.
“Gray-matter loss during child development is part of healthy maturation,” said University of Illinois postdoctoral researcher Laura Chaddock-Heyman, who led the research. “Gray-matter thinning is the sculpting of a fully formed, healthy brain. The theory is that the brain is pruning away unnecessary connections and strengthening useful connections.”
Previous studies have shown that gray-matter thinning is associated with better reasoning and thinking skills, Chaddock-Heyman said.
Role of aerobic fitness in math skills
“We show, for the first time, that aerobic fitness may play a role in this cortical thinning,” she said. “In particular, we find that higher-fit 9- and 10-year-olds show a decrease in gray-matter thickness in some areas known to change with development, specifically in the frontal, temporal and occipital lobes of the brain.”
The analysis included 48 children, all of whom had completed a maximal oxygen-uptake fitness test on a treadmill. Half of the children (the higher-fit kids) were at or above the 70th percentile for aerobic fitness, and half (the lower-fit kids) were at or below the 30th percentile. The researchers imaged the children’s brains using fMRI, and tested their math, reading, and spelling skills using the Wide Range Achievement Test-3, which correlates closely with academic achievement in these fields.
The team found differences in math skills and cortical brain structure between the higher-fit and lower-fit children: thinner gray matter corresponded to better math performance in the higher-fit kids. But they did not find significant fitness-associated differences in reading or spelling aptitude.
So why only math? “Successful mathematics problem solving is said to involve working memory, the ability to hold relevant information in mind for efficient and effective comprehension, as well as inhibition, the ability to ignore irrelevant information,” Chaddock-Heyman explained to KurzweilAI.
“Higher-fit children have shown superior performance on cognitive control tasks that challenge working memory and inhibitory control, relative to lower fit children. Other studies suggest superior performance on standardized tests of mathematics and reading in higher-fit children.”
“These findings arrive at an important time. Physical activity opportunities during the school day are being reduced or eliminated in response to mandates for increased academic time,” according to kinesiology and community health professor Charles H. Hillman. “Given that rates of physical inactivity are rising, there is an increased need to promote physical activity. Schools are the best institutions to implement such health behavior practices, due to the number of children they reach on a daily basis.”
“Future efforts should be directed toward determining whether these biomarkers predict performance on select academic subjects, as suggested in our study, or whether they serve as a more global index of overall school performance.”
The researchers next plan a longitudinal study of children participating in a physical activity training program. The goal is to establish additional neural biomarkers for scholastic success, based on a causal relationship between brain changes, changes in physical fitness, and changes in cognition, and to determine whether these biomarkers predict performance on select academic subjects (as in the current study), or overall school achievement .
The National Institute on Aging, the National Institute of Child Health and Human Development, and the National Institute of Diabetes and Digestive and Kidney Diseases at the National Institutes of Health supported this research. The National Institute of Food and Agriculture at the U.S. Department of Agriculture also provided funding.
Abstract of The Role of Aerobic Fitness in Cortical Thickness and Mathematics Achievement in Preadolescent Children
Growing evidence suggests that aerobic fitness benefits the brain and cognition during childhood. The present study is the first to explore cortical brain structure of higher fit and lower fit 9- and 10-year-old children, and how aerobic fitness and cortical thickness relate to academic achievement. We demonstrate that higher fit children (>70th percentile VO2max) showed decreased gray matter thickness in superior frontal cortex, superior temporal areas, and lateral occipital cortex, coupled with better mathematics achievement, compared to lower fit children (<30th percentile VO2max). Furthermore, cortical gray matter thinning in anterior and superior frontal areas was associated with superior arithmetic performance. Together, these data add to our knowledge of the biological markers of school achievement, particularly mathematics achievement, and raise the possibility that individual differences in aerobic fitness play an important role in cortical gray matter thinning during brain maturation. The establishment of predictors of academic performance is key to helping educators focus on interventions to maximize learning and success across the lifespan.