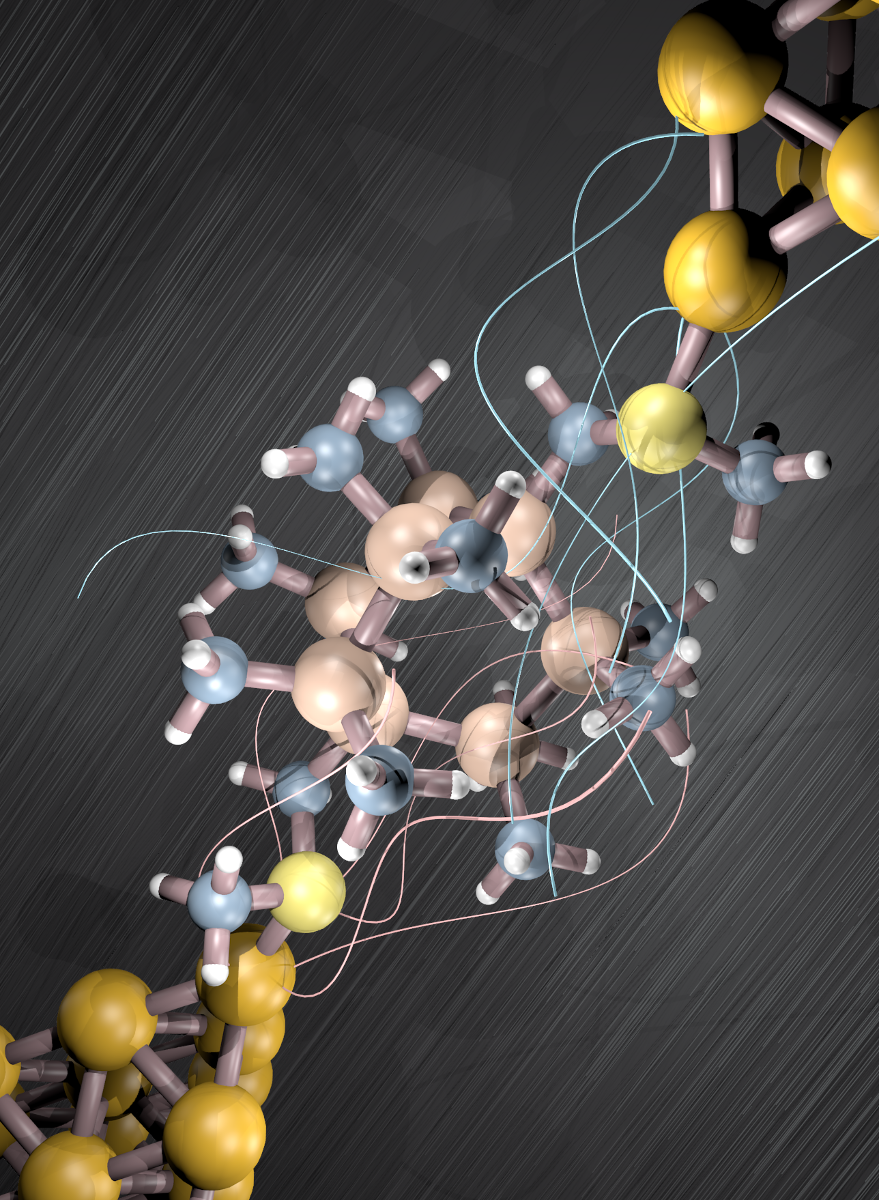
Dysprosium atoms (green) on the surface of nanoparticles can be magnetized in one of two possible directions: “spin up” or “spin down.” (credit: ETH Zurich / Université de Rennes)
Imagine you could store a bit on a single atom or small molecule — the ultimate magnetic data-storage system. An international team of researchers led by chemists from ETH Zurich has taken a step toward that idea by depositing single magnetizable atoms onto a silica surface, with the atoms retaining their magnetism.
In theory, certain atoms can be magnetized in one of two possible directions: “spin up” or “spin down” (representing zero or one); information could then be stored and read based on the sequence of the molecules’ magnetic spin directions. But finding molecules that can store the magnetic information permanently is a challenge, and it’s even more difficult to arrange these molecules on a solid surface to build actual data storage devices.
Magnetizing atoms on nanoparticles
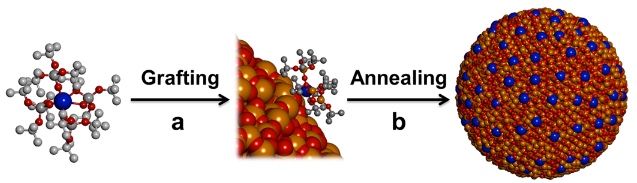
Strategy for immobilization of dysprosium atoms (blue, surrounded by molecular scaffold) on a silica nanoparticle surface, based on a grafting step (a) and a thermolytic (chemical decomposition caused by heat) step (b) (credit: Florian Allouche et al./ ACS Central Science)
Nonetheless, Christophe Copéret, a professor at the Laboratory of Inorganic Chemistry at ETH Zurich, and his team have developed a method using a dysprosium atom (dysprosium is a metal belonging to the rare-earth elements). The atom is surrounded by a molecular scaffold that serves as a vehicle. The scientists also developed a method for depositing such molecules on the surface of silica nanoparticles and fusing them by annealing (heating) at 400 degrees Celsius.
The scaffold molecular structure disintegrates in the process, yielding nanoparticles with dysprosium atoms well-dispersed at the surface. The scientists showed that these atoms can then be magnetized and that they maintain their magnetic information.
One advantage of their new method is its simplicity. Nanoparticles bonded with dysprosium can be made in any chemical laboratory. No cleanroom and complex equipment required. And the magnetizable nanoparticles can be stored at room temperature and re-utilized.
Their magnetization process currently only works at around minus 270 degrees Celsius (near absolute zero), and the magnetization can only be maintained for up to one and a half minutes. So the scientists are now looking for methods that will allow the magnetization to be stabilized at higher temperatures and for longer periods of time. They are also looking for ways to fuse atoms to a flat surface instead of to spherical nanoparticles.
Other preparation methods also involve direct deposition of individual atoms onto a surface, but the materials are only stable at very low temperatures, mainly due to the agglomeration of these individual atoms. Alternatively, molecules with ideal magnetic properties can be deposited onto a surface, but this immobilization often negatively affects the structure and the magnetic properties of the final object.
Scientists from the Universities of Lyon and Rennes, Collège de France in Paris, Paul Scherrer Institute in Switzerland, and Berkeley National Laboratory were involved in the research.
Abstract of Magnetic Memory from Site Isolated Dy(III) on Silica Materials
Achieving magnetic remanence at single isolated metal sites dispersed at the surface of a solid matrix has been envisioned as a key step toward information storage and processing in the smallest unit of matter. Here, we show that isolated Dy(III) sites distributed at the surface of silica nanoparticles, prepared with a simple and scalable two-step process, show magnetic remanence and display a hysteresis loop open at liquid 4He temperature, in contrast to the molecular precursor which does not display any magnetic memory. This singular behavior is achieved through the controlled grafting of a tailored Dy(III) siloxide complex on partially dehydroxylated silica nanoparticles followed by thermal annealing. This approach allows control of the density and the structure of isolated, “bare” Dy(III) sites bound to the silica surface. During the process, all organic fragments are removed, leaving the surface as the sole ligand, promoting magnetic remanence.