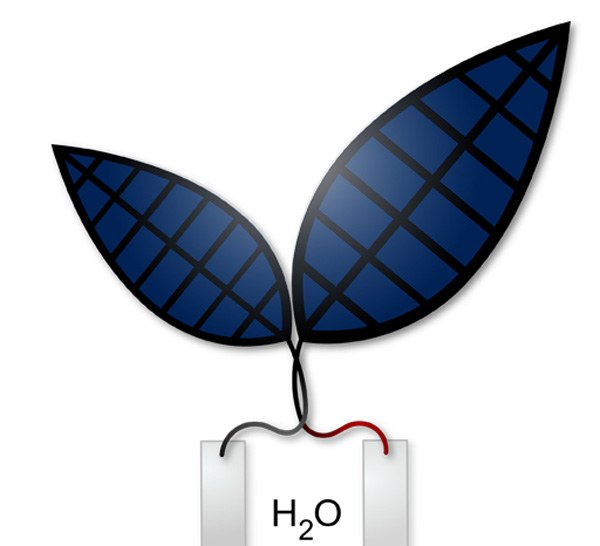
Bionic leaf 2.0: An artificial photosynthesis system (credit: Jessica Polka)
Harvard scientists have created a system a system that uses solar energy plus hydrogen-eating bacteria to produce liquid fuels with 10 percent efficiency, compared to the 1 percent seen in the fastest-growing plants.
The system, co-created by Daniel Nocera, the Patterson Rockwood Professor of Energy at Harvard University, and Pamela Silver, the Elliott T. and Onie H. Adams Professor of Biochemistry and Systems Biology at Harvard Medical School, uses solar energy to split water molecules into hydrogen and oxygen molecules.
A paper on the research was published June 3 in Science.
“This is a true artificial photosynthesis system,” Nocera said. “Before, people were using artificial photosynthesis for water-splitting, but this is a true A-to-Z system, and we’ve gone well over the efficiency of photosynthesis in nature.”
“What we’ve invented is an artificial leaf. You just drop it in water and sunlight hits it, and out one side comes hydrogen and out the other side comes oxygen.” — Daniel Nocera
“The beauty of biology is it’s the world’s greatest chemist: Biology can do chemistry we can’t do easily,” said Silver, who is also a founding core member of the Wyss Institute at Harvard University. “In principle, we have a platform that can make any downstream carbon-based molecule. So this has the potential to be incredibly versatile.”
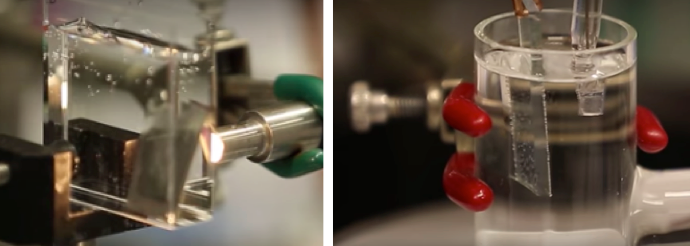
Dubbed “bionic leaf 2.0,” the new system builds on previous work by Nocera, Silver and others, which faced a number of challenges. Mainly, the catalyst they used to produce hydrogen (a nickel-molybdenum-zinc alloy) also created reactive oxygen species — molecules that attacked and destroyed the bacteria’s DNA. To avoid that problem, researchers were forced to run the system at abnormally high voltages, resulting in reduced efficiency.
Ready for commercial applications, with a new model
“For this paper, we designed a new cobalt-phosphorus alloy catalyst, which we showed does not make reactive oxygen species,” Nocera said. “That allowed us to lower the voltage, and that led to a dramatic increase in efficiency.”
Nocera and colleagues were also able to expand the portfolio of the system to include isobutanol (a solvent) and isopentanol (used in geothermal power production to drive turbines), along with PHB, a bioplastic precursor.

“Instead of having a gas station, the Sun is hitting your house, you have the artificial leaf, you could be generating your own fuel.” — Daniel Nocera (credit: Rose Lincoln/Harvard Staff Photographer)
The new catalyst’s chemical design also allows it to “self-heal,” meaning it won’t leach material into solution — it’s biologically compatible.
Nocera said the system is already effective enough to consider possible commercial applications but within a different model for technology translation. “It’s an important discovery… [that] can do better than photosynthesis,” Nocera said. “But I also want to bring this technology to the developing world.”
Working in conjunction with the First 100 Watts Project at Harvard, which helped fund the research, Nocera hopes to continue developing the technology and its applications in nations such as India with the help of that country’s scientists.
In many ways, Nocera said, the new system marks fulfillment of the promise of his “artificial leaf,” which used solar power to split water and make hydrogen fuel (see ‘Artificial leaf’ harnesses sunlight for efficient, safe hydrogen fuel production).
“If you think about it, photosynthesis is amazing,” he said. “It takes sunlight, water and air—and then look at a tree. That’s exactly what we did, but we do it significantly better, because we turn all that energy into a fuel.”
The work, a direct result of the First 100 Watts Project established at Harvard University, was was supported by Office of Naval Research Multidisciplinary University, Research Initiative Award, Air Force Office of Scientific Research Grant, and the Wyss Institute for Biologically Inspired Engineering. The Harvard University Climate Change Solutions Fund is supporting ongoing research into the “bionic leaf” platform.
Harvard University | Bionic Leaf Turns Sunlight Into Liquid Fuel
Abstract of Water splitting–biosynthetic system with CO2 reduction efficiencies exceeding photosynthesis
Artificial photosynthetic systems can store solar energy and chemically reduce CO2. We developed a hybrid water splitting–biosynthetic system based on a biocompatible Earth-abundant inorganic catalyst system to split water into molecular hydrogen and oxygen (H2 and O2) at low driving voltages. When grown in contact with these catalysts, Ralstonia eutropha consumed the produced H2 to synthesize biomass and fuels or chemical products from low CO2 concentration in the presence of O2. This scalable system has a CO2 reduction energy efficiency of ~50% when producing bacterial biomass and liquid fusel alcohols, scrubbing 180 grams of CO2 per kilowatt-hour of electricity. Coupling this hybrid device to existing photovoltaic systems would yield a CO2 reduction energy efficiency of ~10%, exceeding that of natural photosynthetic systems.