
The cloud of methane is temporarily controlled, and a permanent fix is on the way.
The post SoCalGas Plugs the Massive Porter Ranch Methane Leak—For Now appeared first on WIRED.

Science and reality

The cloud of methane is temporarily controlled, and a permanent fix is on the way.
The post SoCalGas Plugs the Massive Porter Ranch Methane Leak—For Now appeared first on WIRED.

In so-called mechanical doping, a rider hides an electric motor in a bike. How could you detect these motors without taking the bike apart?
The post Clever Ways to Catch a Pro Cyclist Cheating With a Hidden Motor appeared first on WIRED.
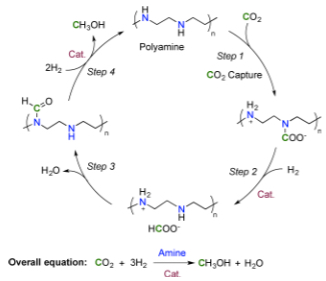
Researchers at the University of Southern California (USC) Loker Hydrocarbon Research Institute have created fuel out of thin air — directly converting carbon dioxide from air into methanol at relatively low temperatures for the first time. While methanol can’t currently compete with oil, it will be there when we run out of oil, the researchers note.
The researchers bubbled air through an aqueous solution of pentaethylenehexamine (PEHA), adding a Ru-Macho-BH ruthenium catalyst to encourage hydrogen to latch onto the CO2 under pressure. They then heated the solution, converting 79 percent of the CO2 into methanol.
Though mixed with water, the resulting methanol can be easily distilled, said G.K. Surya Prakash, professor of chemistry and director of the Loker Hydrocarbon Research Institute.
Proposed reaction sequence for CO2 capture and in situ hydrogenation to methanol (credit: J. Kothandaraman et al./Journal of the American Chemical Society)
Scaling up
Methanol (aka “wood alcohol” or “rubbing alcohol”) is attractive because it can be directly used as a clean-burning liquid fuel for internal combustion engines and for fuel cells. It’s also a hydrogen storage medium and a chemical feedstock for producing a myriad of chemicals and products, including ethylene and propylene. It’s one of the most important building blocks in the chemical industry, with an annual production of more than 70 million tons.
The research is part of a broader effort to use renewable energy to transform greenhouse gas into its combustible form — attacking global warming from two angles simultaneously.
Prakash and Olah hope to refine the process to the point that it could be scaled up for industrial use within five to 10 years. “Of course it won’t compete with oil today, at around $30 per barrel,” Prakash said. “But right now we burn fossilized sunshine. We will run out of oil and gas, but the sun will be there for another five billion years. So we need to be better at taking advantage of it as a resource.”
Lower temperatures
Previous efforts have required a slower multistage process with the use of high temperatures and high concentrations of CO2, meaning that renewable energy sources would not be able to efficiently power the process.
The new system operates at around 125 to 165 degrees Celsius (257 to 359 degrees Fahrenheit), minimizing the decomposition of the catalyst — which occurs at 155 degrees Celsius (311 degrees Fahrenheit). The system uses a homogeneous catalyst, making it a quicker “one-pot” process. In a lab, the researchers demonstrated that they were able to run the process five times with only minimal loss of the effectiveness of the catalyst.
The new process was published in the Journal of the American Chemical Society on Dec. 29. The research was supported by the USC Loker Hydrocarbon Research Institute.
Abstract of Conversion of CO2 from Air into Methanol Using a Polyamine and a Homogeneous Ruthenium Catalyst
A highly efficient homogeneous catalyst system for the production of CH3OH from CO2 using pentaethylenehexamine and Ru-Macho-BH (1) at 125–165 °C in an ethereal solvent has been developed (initial turnover frequency = 70 h–1 at 145 °C). Ease of separation of CH3OH is demonstrated by simple distillation from the reaction mixture. The robustness of the catalytic system was shown by recycling the catalyst over five runs without significant loss of activity (turnover number > 2000). Various sources of CO2 can be used for this reaction including air, despite its low CO2 concentration (400 ppm). For the first time, we have demonstrated that CO2 captured from air can be directly converted to CH3OH in 79% yield using a homogeneous catalytic system.

Flying over Aliso Canyon provided the only available estimates of rate of the leak, which has been as high as 145,000 pounds per hour.
The post The Lone Pilot Flying Over California’s Giant Methane Leak appeared first on WIRED.

“Solar thermal fuel” polymer film comprising three distinct layers with tunable thickness (4 to 5 microns for each) (credit: Courtesy of the researchers)
MIT researchers have developed a new transparent polymer film that can store solar energy during the day and release it later as heat, whenever needed. The material could be applied to many different surfaces, such as window glass or clothing.
The new material solves a problem with renewable solar energy: the Sun is not available at night or on stormy days. Most solutions have focused on storing and recovering solar energy as electricity or other forms. The new finding could provide a highly efficient method for storing the sun’s energy through a chemical storage system, which can retain the energy indefinitely in a stable molecular configuration and release it later as heat.
Storing-releasing heat as molecular configurations
The finding, by a research team headed by MIT professor Jeffrey Grossman, is described in a paper in the journal Advanced Energy Materials.
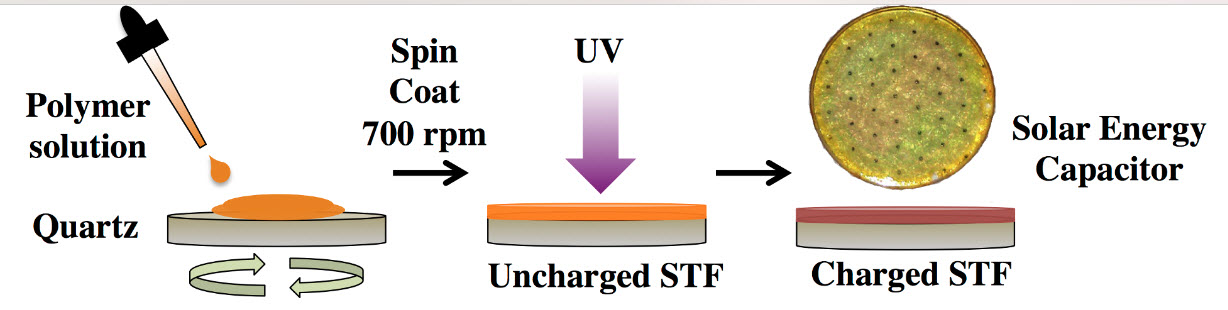
A spin-coating process enables the solar thermal fuel polymer material to deposit from solution. The film can then be readily charged with ultraviolet light. This process can be extended to a variable-thickness layer-by-layer process. (credit: Courtesy of the researchers)
The key is an azobenzene molecule that can remain stable in either of two different configurations: charged and uncharged. When exposed to sunlight, the energy of the light kicks the molecules into their “charged” configuration, and they can stay that way for long periods. Then, when triggered by a very specific temperature or other stimulus, the molecules snap back to their original shape, giving off a burst of heat in the process.
Built-in windshield de-icing

The platform for testing macroscopic heat release. A heating element (bottom) is used to provide sufficient energy to trigger the solar thermal fuel materials, while an infrared camera (yellow circles) monitors the temperature. The charged film (right) releases heat enabling a higher temperature relative to the uncharged film (left). (credit: Courtesy of the researchers)
The “solar thermal fuel” material is highly transparent, which could make it useful for de-icing car windshields, says Grossman, the Morton and Claire Goulder and Family Professor in Environmental Systems and a professor of materials science and engineering.
While many cars already have fine heating wires embedded in rear windows for that purpose, anything that blocks the view through the front window is forbidden by law, even thin wires.
But a transparent film made of the new material, sandwiched between two layers of glass — as is currently done with bonding polymers to prevent pieces of broken glass from flying around in an accident — could provide the same de-icing effect without any blockage. German auto company BMW, a sponsor of this research, is interested in that potential application, Grossman says.
With such a window, energy would be stored in the polymer every time the car sits out in the sunlight. Then, “when you trigger it,” using just a small amount of heat that could be provided by a heating wire or puff of heated air, “you get this blast of heat,” Grossman says.
“We did tests to show you could get enough heat to drop ice off a windshield.” Accomplishing that, he explains, doesn’t require that all the ice actually be melted, just that the ice closest to the glass melts enough to provide a layer of water that releases the rest of the ice to slide off by gravity or be pushed aside by the windshield wipers.
The team is continuing to work on improving the film’s properties, Grossman says, improving its transparency and temperature increase (from 10 degrees Celsius above the surrounding temperature — sufficient for the ice-melting application — to 20 degrees). The new polymer could also significantly reduce electrical drain for heating and de-icing in electric cars, he says.
The work was supported by a NSERC Canada Banting Fellowship and by BMW.
Abstract of Solid-State Solar Thermal Fuels for Heat Release Applications
Closed cycle systems offer an opportunity for solar energy harvesting and storage all within the same material. Photon energy is stored within the chemical conformations of molecules and is retrieved by a triggered release in the form of heat. Until now, such solar thermal fuels (STFs) have been largely unavailable in the solid-state, which would enable them to be utilized for a multitude of applications. A polymer STF storage platform is synthesized employing STFs in the solid-state. This approach enables uniform films capable of appreciable heat storage of up to 30 Wh kg−1 and that can withstand temperature of up to 180 °C. For the first time a macroscopic energy release is demonstrated using spatial infrared heat maps with up to a 10 °C temperature change. These findings pave the way for developing highly efficient and high energy density STFs for applications in the solid-state.

Looking back on the previous seasons of MythBusters, here are five great physics demos.
The post 5 of the Greatest Physics Demos From the MythBusters appeared first on WIRED.

A common idea is that energy is stored in chemical bonds. This isn't really true. Here's why.
The post We Need to Talk About the Energy in Chemical Bonds appeared first on WIRED.

What the Sun might look like if it were to produce a superflare. A large flaring coronal loop structure is shown towering over a solar active region. (credit: University of Warwick/Ronald Warmington)
Astrophysicists have discovered a stellar “superflare” on a star observed by NASA’s Kepler space telescope with wave patterns similar to those that have been observed in the Sun’s solar flares. (Superflares are flares that are thousands of times more powerful than those ever recorded on the Sun, and are frequently observed on some stars.)
The scientists found the evidence in the star KIC9655129 in the Milky Way. They suggest there are similarities between the superflare on KIC9655129 and the Sun’s solar flares, so the underlying physics of the flares might be the same.
Disastrous for life on Earth
Typical solar flares can have energies equivalent to a 100 million megaton bombs, but a superflare on our Sun could release energy equivalent to 100 billion megaton bombs, the scientists say.
The effects on the power grid in the U.S. would be similar to those resulting from a major cyberattack on America’s power grid, as described in the just-published book, Lights Out: A Cyberattack, A Nation Unprepared, Surviving the Aftermath.
The Earth’s communications and energy systems could be at serious risk of failing, the scientists note, and disastrous for life on Earth. Our GPS and radio communication systems could be severely disrupted and there could be large-scale power blackouts as a result of strong electrical currents being induced in power grids.
The evidence
Research co-author Anne-Marie Broomhall, PhD, from the University of Warwick explains: “When a flare occurs, we typically see a rapid increase in intensity followed by a gradual decline. Usually the decline phase is relatively smooth but occasionally there are noticeable bumps, which are termed ‘quasi-periodic pulsations’ or QPPs.”
The scientists used techniques called wavelet analysis and Monte Carlo modeling to assess the periodicity and statistical significance of these QPPs. The analysis revealed two significant periodicities, with less than a 1% probability that these pulsations would be observed by chance. The most plausible explanation for the presence of two independent periodicities: the QPPs were caused by magnetohydrodynamic (MHD) oscillations, which are also frequently observed in solar flares, the scientists say.
“This result is, therefore, an indication that the same physical processes are involved in both solar flares and stellar superflares. The latter finding supports the hypothesis that the Sun is able to produce a potentially devastating superflare.”
(Also see previous KurzweilAI coverage of this subject.)
The research is published by The Astrophysical Journal Letters and was funded by the European Research Council.
Abstract for A Multi-Period Oscillation In A Stellar Superflare
Flares that are orders of magnitude larger than the most energetic solar flares are routinely observed on Sun-like stars, raising the question of whether the same physical processes are responsible for both solar and stellar flares. In this Letter, we present a white-light stellar superflare on the star KIC 9655129, observed by NASA’s Kepler mission, with a rare multi-period quasi-periodic pulsation (QPP) pattern. Two significant periodic processes were detected using the wavelet and autocorrelation techniques, with periods of 78 ± 12 minutes and 32 ± 2 minutes. By comparing the phases and decay times of the two periodicities, the QPP signal was found to most likely be linear, suggesting that the two periodicities are independent, possibly corresponding either to different magnetohydrodynamic (MHD) modes of the flaring region or different spatial harmonics of the same mode. The presence of multiple periodicities is a good indication that the QPPs were caused by MHD oscillations and suggests that the physical processes in operation during stellar flares could be the same as those in solar flares.

Silicon pillars emerge from nanosize holes in a thin gold film. The pillars funnel 97 percent of incoming light to a silicon substrate, a technology that could significantly boost the performance of conventional solar cells. (credit: Vijay Narasimhan, Stanford University)
Stanford scientists have discovered how to make the electrical wiring on top of solar cells nearly invisible to incoming light, using nanosize silicon pillars to hide the wires. The new design could dramatically boost solar-cell efficiency, the researchers suggest.
A solar cell is basically a semiconductor that converts sunlight into electricity, sandwiched between metal contacts that carry the electrical current generated by the cell. But with current designs, the shiny metal wires on top of the cell reflect sunlight away from the semiconductor surface, reducing the cell’s efficiency.
Now Stanford scientists have discovered how to hide the reflective upper contacts, funneling light directly to the semiconductor below by using silicon pillars to redirect the sunlight before it hits the metallic surface.
Stanford University | “Invisible wires” could boost solar-cell efficiency
Besides gold, the nanopillar architecture will also work with contacts made of silver, platinum, nickel and other metals. In addition to silicon, this new technology can be used with other semiconducting materials for a variety of applications, including photosensors, light-emitting diodes and displays and transparent batteries, as well as solar cells.
The new method aims to improve on a wide variety of methods that have been reported by KurzweilAI.
The findings are published in the journal ACS Nano.
Abstract of Hybrid Metal–Semiconductor Nanostructure for Ultrahigh Optical Absorption and Low Electrical Resistance at Optoelectronic Interfaces
Engineered optoelectronic surfaces must control both the flow of light and the flow of electrons at an interface; however, nanostructures for photon and electron management have typically been studied and optimized separately. In this work, we unify these concepts in a new hybrid metal–semiconductor surface that offers both strong light absorption and high electrical conductivity. We use metal-assisted chemical etching to nanostructure the surface of a silicon wafer, creating an array of silicon nanopillars protruding through holes in a gold film. When coated with a silicon nitride anti-reflection layer, we observe broad-band absorption of up to 97% in this structure, which is remarkable considering that metal covers 60% of the top surface. We use optical simulations to show that Mie-like resonances in the nanopillars funnel light around the metal layer and into the substrate, rendering the metal nearly transparent to the incoming light. Our results show that, across a wide parameter space, hybrid metal–semiconductor surfaces with absorption above 90% and sheet resistance below 20 Ω/□ are realizable, suggesting a new paradigm wherein transparent electrodes and photon management textures are designed and fabricated together to create high-performance optoelectronic interfaces.

In the trailer for The Good Dinosaur, we see an asteroid zooming past the Earth. How fast is this asteroid?
The post The Asteroid in The Good Dinosaur Travels at Half the Speed of Light appeared first on WIRED.