
India will make a choice, but it will not be India's alone.
The post Solar or Coal? The Energy India Picks May Decide Earth’s Fate appeared first on WIRED.

Science and reality

India will make a choice, but it will not be India's alone.
The post Solar or Coal? The Energy India Picks May Decide Earth’s Fate appeared first on WIRED.

The New Shepard spacecraft plans to launch people into space. However, going into space is different than getting into orbit.
The post It’d Be Way Harder for Bezos’ Rocket to Get Into Orbit Than Just Space appeared first on WIRED.

Using wind, water, and solar electricity for everything, instead of burning fuel, with resulting improved energy efficiency, means you need much less energy (credit: The Solutions Project/data: Stanford University)
Stanford and UC Berkeley researchers have a solution to the problem of storing energy from wind, water and solar power overnight (or in inclement weather): store it underground. The system could result in a reliable, affordable national grid, replacing fossil fuel, they believe.
How it would work
This methodology for keeping the grid stable should work in many places worldwide, say Mark Z. Jacobson, a Stanford professor of civil and environmental engineering, Mark Delucchi of the University of California, Berkeley, and associates. They describe the plan today (Nov. 23) in Proceedings of the National Academy of Sciences.
According to the authors, in the U.S., for example, the resulting drop in air pollution would save tens of thousands of lives each year (up to 65,000 people die prematurely in America annually as a result of air pollution) and the plan could result in about 5 million 40-year jobs.
Abstract of A low-cost solution to the grid reliability problem with 100% penetration of intermittent wind, water, and solar for all purposes
This study addresses the greatest concern facing the large-scale integration of wind, water, and solar (WWS) into a power grid: the high cost of avoiding load loss caused by WWS variability and Q:10 uncertainty. It uses a new grid integration model and finds lowcost, no-load-loss, nonunique solutions to this problem on electrification of all US energy sectors (electricity, transportation, heating/cooling, and industry) while accounting for wind and solar time series data from a 3D global weather model that simulates extreme events and competition among wind turbines for available kinetic energy. Solutions are obtained by prioritizing storage for heat (in soil and water); cold (in ice and water); and electricity (in phase-change materials, pumped hydro, hydropower, and hydrogen), and using demand response. No natural gas, biofuels, or stationary batteries are needed. The resulting 2050–2055 US electricity social cost for a full systems is much less than for fossil fuels. These results hold for many conditions, suggesting that low-cost, stable 100% WWS systems should work many places worldwide.

What do a humpback and a wind turbine have in common? Scalloped blade edges.
The post Humpback Whales Solve a Big Problem for Wind Turbines appeared first on WIRED.
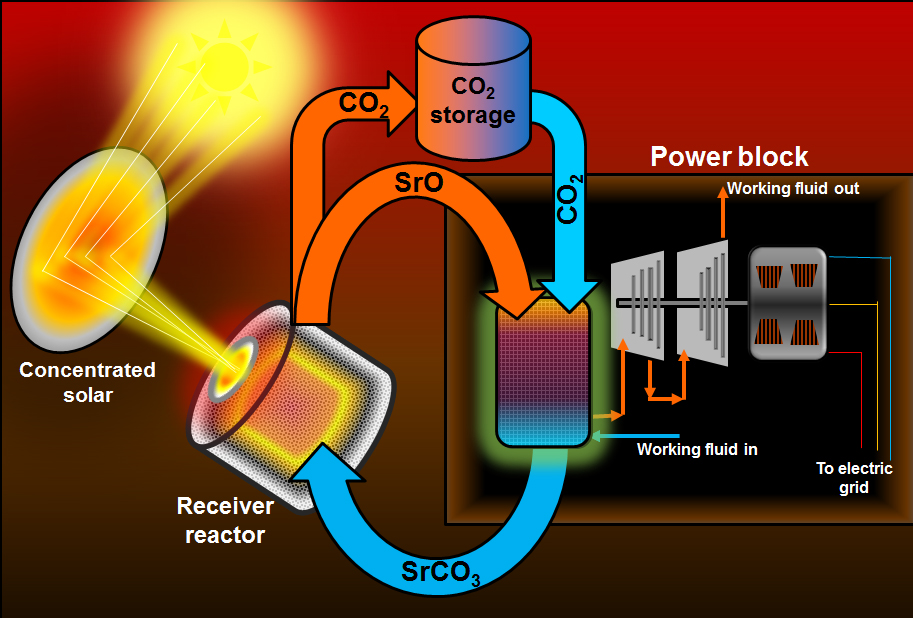
An advance in the storage of concentrated solar thermal energy may reduce reduce its cost and make it more practical to supply 24-hour on-demand electrical power (credit: Kelvin Randhir, courtesy of the University of Florida)
Oregon State University (OSU) engineers have developed an innovation in chemical storage of concentrated solar thermal energy that may reduce its cost and make it more practical for wider use.
The new system uses thermochemical storage, in which chemical transformation is used in repeated cycles to hold heat, use it to drive turbines to create electricity, and then be re-heated to continue the cycle. Most commonly, this might be done over a 24-hour period, with variable levels of solar-powered electricity available at any time of day, as dictated by demand.
Unlike conventional solar photovoltaic cells, concentrated solar thermal (a.k.a. concentrated solar power, or CSP) uses huge arrays of mirrors to focus light, typically onto a tower, for temporarily storing the energy, which is more cost-effective than batteries. (See Australian researchers set new world record in solar-energy efficiency.)

The PS10 Solar Power Plant in Spain concentrates sunlight from a field of 624 heliostats (movable mirrors) onto a central solar power tower, generating 11 megawatts (credit: Abengoa Solar, S.A.)
Storage of this type helps eliminate one of the key factors limiting the wider use of solar energy: The need to deliver the electricity immediately.
A ten-fold increase in energy density and twice as efficient
The OSU development overcomes a limitation in thermochemical energy storage. “In these types of systems, energy efficiency is closely related to use of the highest temperatures possible,” said Nick AuYeung, an assistant professor of chemical engineering in the OSU College of Engineering and corresponding author of a paper in ChemSusChem, a professional journal covering sustainable chemistry.
Thermochemical storage functions like a battery, in which chemical bonds are used to store and release heat (not electrical) energy. However, the molten salts now being used to store solar thermal energy can only work at about 600 degrees centigrade, and also require large containers and corrosive materials, he explained. “The compound we’re studying can be used at up to 1,200 degrees, and might be twice as efficient as existing systems. There’s a significant potential to lower costs and increase efficiency.”
AuYeung said the new OSU system is based on the reversible decomposition of strontium carbonate into strontium oxide and carbon dioxide, which consumes thermal energy. During discharge, the recombination of strontium oxide and carbon dioxide releases the stored heat. These materials are nonflammable, readily available, and environmentally safe.
In comparison to existing thermochemical approaches, the new system could also allow a ten-fold increase in energy density (energy storage per unit volume), and it’s physically much smaller and would be cheaper to build. The proposed system could first be used to directly heat air, which would drive a turbine to produce electricity, and then residual heat could be used to make steam to drive yet another turbine.
However, in laboratory tests, the current energy storage capacity of the process declined after 45 heating and cooling cycles, due to some changes in the underlying materials. Further research will be needed to identify ways to reprocess the materials or significantly extend the number of cycles that could be performed before any reprocessing was needed, AuYeung said.
Other refinements may also be necessary to test the system at larger scales and resolve issues such as thermal shocks, he said, before a prototype could be ready for testing at a national laboratory.
The work was supported by the SunShot Initiative of the U.S. Department of Energy, and done in collaboration with researchers at the University of Florida.
Abstract of Solar Thermochemical Energy Storage Through Carbonation Cycles of SrCO3/SrO Supported on SrZrO3
Solar thermochemical energy storage has enormous potential for enabling cost-effective concentrated solar power (CSP). A thermochemical storage system based on a SrO/SrCO3 carbonation cycle offers the ability to store and release high temperature (≈1200 °C) heat. The energy density of SrCO3/SrO systems supported by zirconia-based sintering inhibitors was investigated for 15 cycles of exothermic carbonation at 1150 °C followed by decomposition at 1235 °C. A sample with 40 wt % of SrO supported by yttria-stabilized zirconia (YSZ) shows good energy storage stability at 1450 MJ m−3 over fifteen cycles at the same cycling temperatures. After further testing over 45 cycles, a decrease in energy storage capacity to 1260 MJ m−3 is observed during the final cycle. The decrease is due to slowing carbonation kinetics, and the original value of energy density may be obtained by lengthening the carbonation steps.

Schematic of electrode process design. (a) Components mixing under ultrasonic irradiation, (b) an optical image of the as-fabricated electrode made of silicon nanoparticles (SiNP), sulpher-doped graphene (SG), and polyacrylonitrile (PAN), (c) the electrode after sluggish heat treatment (SHT), (d) Schematic of the atomic-scale structure of the electrode. (credit: Fathy M. Hassan et al./Nature Communications)
Zhongwei Chen, a chemical engineering professor at the University of Waterloo, and a team of graduate students have created a new low-cost battery design using silicon instead of graphite, boosting the performance and life of lithium-ion batteries.
Waterloo’s silicon battery technology promises a 40 to 60 per cent increase in energy density (energy storage per unit volume), which is important for consumers with smartphones, smart homes, and smart wearables. It also means an electric car could be driven up to 500 kilometers (311 miles) between charges while reducing its overall weight.
The graphite bottleneck
The Waterloo engineers found that silicon anode materials are capable of producing batteries that store almost 10 times more energy than with graphite.
“As batteries improve, graphite is slowly becoming a performance bottleneck because of the limited amount of energy that it can store,” said Chen, the Canada Research Chair in Advanced Materials for Clean Energy and a member of the Waterloo Institute for Nanotechnology and the Waterloo Institute for Sustainable Energy.
The most critical challenge the Waterloo researchers faced in the new design was the loss of energy that occurs when silicon contracts and then expands by as much as 300 per cent with each charge cycle. The resulting increase and decrease in silicon volume forms cracks that reduce battery performance, create short circuits, and eventually cause the battery to stop operating.
To overcome this problem, Chen’s team along with the General Motors Global Research and Development Centre developed a flash heat treatment for fabricated silicon-based lithium-ion electrodes that minimizes volume expansion while boosting the performance and cycle capability of lithium-ion batteries.
“The economical flash heat treatment creates uniquely structured silicon anode materials that deliver extended cycle life to more than 2000 cycles with increased energy capacity of the battery,” said Chen.
Chen expects to see new batteries based on the design on the market next year.
Their findings are published in an open-access paper in the latest issue of Nature Communications.
Abstract of Evidence of covalent synergy in silicon–sulfur–graphene yielding highly efficient and long-life lithium-ion batteries
Silicon has the potential to revolutionize the energy storage capacities of lithium-ion batteries to meet the ever increasing power demands of next generation technologies. To avoid the operational stability problems of silicon-based anodes, we propose synergistic physicochemical alteration of electrode structures during their design. This capitalizes on covalent interaction of Si nanoparticles with sulfur-doped graphene and with cyclized polyacrylonitrile to provide a robust nanoarchitecture. This hierarchical structure stabilized the solid electrolyte interphase leading to superior reversible capacity of over 1,000 mAh g−1 for 2,275 cycles at 2 A g−1. Furthermore, the nanoarchitectured design lowered the contact of the electrolyte to the electrode leading to not only high coulombic efficiency of 99.9% but also maintaining high stability even with high electrode loading associated with 3.4 mAh cm−2. The excellent performance combined with the simplistic, scalable and non-hazardous approach render the process as a very promising candidate for Li-ion battery technology.

A new catalyst just 15 microns thick has proven nearly as effective as platinum-based catalysts but at a much lower cost, according to scientists at Rice University. The catalyst is made of nitrogen-doped graphene with individual cobalt atoms that activate the process. (credit: Tour Group/Rice University)
Graphene doped with nitrogen and augmented with cobalt atoms has proven to be an effective, durable catalyst for the production of hydrogen from water, according to scientists at Rice University.
The Rice University lab of chemist James Tour and colleagues has developed a robust, solid-state catalyst that shows promise to replace expensive platinum for hydrogen generation. (Catalysts can split water into its constituent hydrogen and oxygen atoms, a process required for fuel cells.)
The latest discovery, detailed in Nature Communications, is a significant step toward lower-cost catalysts for energy production, according to the researchers.

Disordered graphitic carbon doped with nitrogen and augmented with cobalt atoms serves as an efficient, robust catalyst for hydrogen separation from water. The material discovered at Rice University could challenge more expensive platinum-based catalysts. (credit: Tour Group/Rice University)
Cost-effective replacement for platinum
“What’s unique about this paper is that we show … the use of atoms,” Tour said, instead of the conventional use of metal particles or nanoparticles. “The particles doing this chemistry are as small as you can possibly get.”
Even particles on the nanoscale work only at the surface, he explained. “There are so many atoms inside the nanoparticle that never do anything. But in our process, the atoms driving catalysis have no metal atoms next to them. We’re getting away with very little cobalt to make a catalyst that nearly matches the best platinum catalysts.” He said that in comparison tests, the new material nearly matched platinum’s efficiency to begin reacting at a low onset voltage (the amount of electricity it needs to begin separating water into hydrogen and oxygen).
The researchers discovered that heat-treating graphene oxide and small amounts of cobalt salts in a gaseous environment forced individual cobalt atoms to bind to the material. Electron microscope images showed cobalt atoms widely dispersed throughout the samples. They also tested nitrogen-doped graphene on its own and found it lacked the ability to kick the catalytic process into gear. But adding cobalt in very small amounts significantly increased its ability to split acidic or basic water.
The new catalyst is mixed as a solution and can be reduced to a paper-like material or used as a surface coating. Tour said single-atom catalysts have been realized in liquids, but rarely on a surface. “This way we can build electrodes out of it,” he said. “It should be easy to integrate into devices.”

Cobalt atoms shine in an electron microscope image of a new catalyst for hydrogen production invented at Rice University. The widely separated cobalt atoms are bound to a sheet of nitrogen-doped graphene. (credit: Tour Group/Rice University)
“This is an extremely high-performance material,” Tour added. He noted platinum-carbon catalysts still boast the lowest onset voltage. “No question, they’re the best. But this is very close to it and much easier to produce and hundreds of times less expensive.”
Atom-thick graphene is the ideal substrate, Tour said, because of its high surface area, stability in harsh operating conditions, and high conductivity. Samples of the new catalyst showed a negligible decrease in activity after 10 hours of accelerated degradation studies in the lab.
Rice colleagues at the Chinese Academy of Sciences, the University of Texas at San Antonio, and the University of Houston were also involved in the research.
Rice University | H2 evolution
Reduction of water to hydrogen through electrocatalysis holds great promise for clean energy, but its large-scale application relies on the development of inexpensive and efficient catalysts to replace precious platinum catalysts. Here we report an electrocatalyst for hydrogen generation based on very small amounts of cobalt dispersed as individual atoms on nitrogen-doped graphene. This catalyst is robust and highly active in aqueous media with very low overpotentials (30 mV). A variety of analytical techniques and electrochemical measurements suggest that the catalytically active sites are associated with the metal centres coordinated to nitrogen. This unusual atomic constitution of supported metals is suggestive of a new approach to preparing extremely efficient single-atom catalysts.
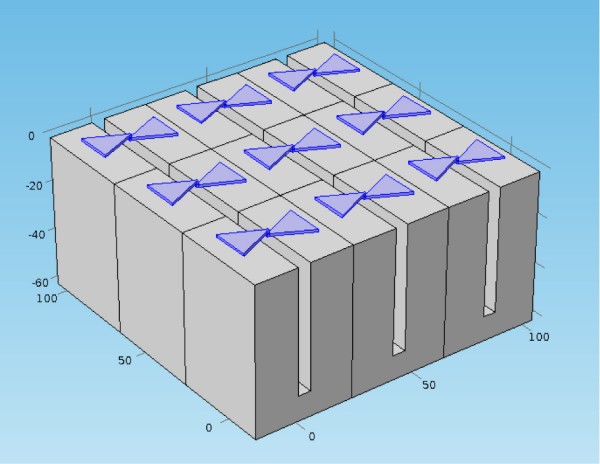
A rectenna metamaterial surface with bow-tie antennas for capture 10,000 to 100,000 times more thermal energy for conversion to DC electricity (credit: Won Park/University of Colorado)
Scientists from the University of Colorado are developing a new type of “rectenna” to efficiently “harvest” thermal emissions (waste heat) radiated from devices (a rectenna converts electromagnetic radiation to DC current).
Currently rectennas work best at low frequencies, but most heat is at higher radiation frequencies — up to the 100 THz (100 trillion cycles per second) range. So Won Park and his colleagues found a way to enhance thermal emission of hot bodies at the lower end of the spectrum (around 1 THz): by manipulating the surface of the object.
A metamaterial for engineering thermal emission
Park’s team uses software to analyze how the nanoscale topology of a surface — its bumps, holes or grooves — changes the way that electromagnetic radiation interacts with the surface. In some instances the geometry supports the formation of a wave of rippling electronic charges, called a plasmon, that hugs the surface.
“We design the surface to support a surface wave, because the presence of the wave offers a new avenue for engineering thermal emission,” Park said. For the case of optimizing thermal energy harvesting, the researchers found they could “spectrally tune” a surface to emit more radiation at 1 THz frequency.
The researchers first optimized the design, which consists of a copper plate with a regular array of tiny holes, using simulations. They then built the design in the lab and confirmed that the plate did indeed produce the type of surface waves predicted by the simulations.
The researchers also used computer modeling to design a bowtie-shaped antenna that would effectively capture the enhanced thermal emission. Simulations predict that an antenna placed near the holey surface could capture 10,000 to 100,000 times more thermal energy than an antenna in open space.
The team is in the process of experimentally testing this prediction and hopes to have new results to report soon. The results will also help the team calculate how rectenna thermal energy harvesting might compare to other ways of harvesting waste heat, such as thermoelectric materials.
The researchers described the system at the AVS 62nd International Symposium and Exhibition in San Jose, Calif. today (Monday, Oct. 19). The research is funded in part by a grant from Redwave Energy Inc.
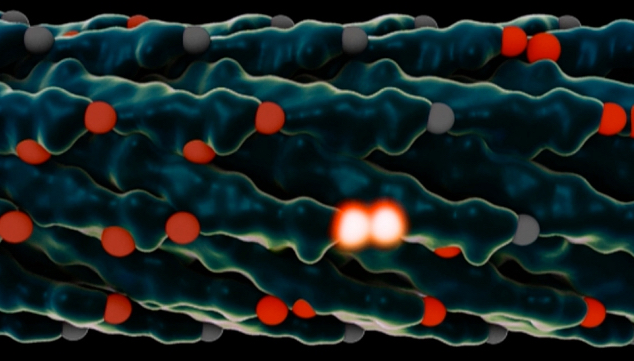
Rendering of a virus used in the MIT experiments. The light-collecting centers, called chromophores, are in red, and chromophores that just absorbed a photon of light are glowing white. After the virus is modified to adjust the spacing between the chromophores, energy can jump from one set of chromophores to the next faster and more efficiently. (credit: the researchers and Lauren Alexa Kaye)
MIT engineers have achieved a significant efficiency boost in a light-harvesting system, using genetically engineered viruses to achieve higher efficiency in transporting energy from receptors to reaction centers where it can be harnessed, making use of the exotic effects of quantum mechanics. Emulating photosynthesis in nature, it could lead to inexpensive and efficient solar cells or light-driven catalysis,
This achievement in coupling quantum research and genetic manipulation, described this week in the journal Nature Materials, was the work of MIT professors Angela Belcher, an expert on engineering viruses to carry out energy-related tasks, and Seth Lloyd, an expert on quantum theory and its potential applications, and 15 collaborators at MIT and in Italy.
The “Quantum Goldilocks Effect”
In photosynthesis, a photon hits a receptor called a chromophore, which in turn produces an exciton — a quantum particle of energy. This exciton jumps from one chromophore to another until it reaches a reaction center, where that energy is harnessed to build the molecules that support life, or photosynthesis.
But the hopping pathway of excitons is random and inefficient unless it takes advantage of quantum effects that allow it, in effect, to take multiple pathways at once and select the best ones, behaving more like a wave than a particle.
To do that, the chromophores have to be arranged just right, with exactly the right amount of space between them. This, Lloyd explains, is known as the “Quantum Goldilocks Effect.”
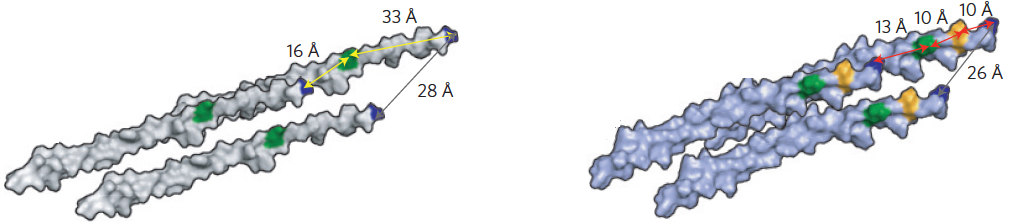
Molecular models of the genetically engineered viruses. Left virus has long inter-binding site distances of 16Å and 33Å within two proteins. Right virus has closer inter-binding site distances of approximately 10Å and 13Å, achieving faster excitation-energy transport speed. (credit: Heechul Park et al./Nature Materials)
That’s where the virus comes in. By engineering a virus that Belcher has worked with for years, the team was able to get it to bond with multiple synthetic chromophores — or, in this case, organic dyes. The researchers were then able to produce many varieties of the virus, with slightly different spacings between those synthetic chromophores, and select the ones that performed best.
In the end, they were able to more than double excitons’ speed, increasing the distance they traveled before dissipating — a significant improvement in the efficiency of the process.
The project started from a chance meeting at a conference in Italy. Lloyd and Belcher, a professor of biological engineering, were reporting on different projects they had worked on, and began discussing the possibility of a project encompassing their very different expertise. Lloyd, whose work is mostly theoretical, pointed out that the viruses Belcher works with have the right length scales to potentially support quantum effects.
In 2008, Lloyd had published a paper demonstrating that photosynthetic organisms transmit light energy efficiently because of these quantum effects. When he saw Belcher’s report on her work with engineered viruses, he wondered if that might provide a way to artificially induce a similar effect, in an effort to approach nature’s efficiency.
“I had been talking about potential systems you could use to demonstrate this effect, and Angela said, ‘We’re already making those,’” Lloyd recalls. Eventually, after much analysis, “We came up with design principles to redesign how the virus is capturing light, and get it to this quantum regime.”
Within two weeks, Belcher’s team had created their first test version of the engineered virus. Many months of work then went into perfecting the receptors and the spacings.
Once the team engineered the viruses, they were able to use laser spectroscopy and dynamical modeling to watch the light-harvesting process in action, and to demonstrate that the new viruses were indeed making use of quantum coherence to enhance the transport of excitons.
“It was really fun,” Belcher says. “A group of us who spoke different [scientific] languages worked closely together, to both make this class of organisms, and analyze the data. That’s why I’m so excited by this.”
Inexpensive and efficient solar cells or light-driven catalysis
While this initial result is essentially a proof of concept rather than a practical system, it points the way toward an approach that could lead to inexpensive and efficient solar cells or light-driven catalysis, the team says. So far, the engineered viruses collect and transport energy from incoming light, but do not yet harness it to produce power (as in solar cells) or molecules (as in photosynthesis). But this could be done by adding a reaction center, where such processing takes place, to the end of the virus where the excitons end up.
“This is exciting and high-quality research,” says Alán Aspuru-Guzik, a professor of chemistry and chemical biology at Harvard University who was not involved in this work. The research, he says, “combines the work of a leader in theory (Lloyd) and a leader in experiment (Belcher) in a truly multidisciplinary and exciting combination that spans biology to physics to potentially, future technology.”
“Access to controllable excitonic systems is a goal shared by many researchers in the field,” Aspuru-Guzik adds. “This work provides fundamental understanding that can allow for the development of devices with an increased control of exciton flow.”
The research was supported by the Italian energy company Eni through the MIT Energy Initiative. The team included researchers at the University of Florence, the University of Perugia, and Eni.
MIT | See how researchers genetically engineer viruses to more efficiently transport energy.
Abstract of Enhanced energy transport in genetically engineered excitonic networks
One of the challenges for achieving efficient exciton transport in solar energy conversion systems is precise structural control of the light-harvesting building blocks. Here, we create a tunable material consisting of a connected chromophore network on an ordered biological virus template. Using genetic engineering, we establish a link between the inter-chromophoric distances and emerging transport properties. The combination of spectroscopy measurements and dynamic modelling enables us to elucidate quantum coherent and classical incoherent energy transport at room temperature. Through genetic modifications, we obtain a significant enhancement of exciton diffusion length of about 68% in an intermediate quantum-classical regime.

An illustration of a tokamak with plasma (credit: ITER Organization)
Fusion reactors could become an economically viable means of generating electricity within a few decades, replacing conventional nuclear power stations, according to new research at Durham University and Culham Centre for Fusion Energy in Oxfordshire, U.K.
The research, published in the journal Fusion Engineering and Design, builds on earlier findings that a fusion power plant could generate electricity at a price similar to that of a fission plant and identifies new advantages in using new superconductor technology.
Such findings support the possibility that, within a generation or two, fusion reactors could offer an almost unlimited supply of energy without contributing to global warming or producing hazardous products on a significant scale.
No radioactive waste or leaks
Fusion reactors generate electricity by heating plasma to around 100 million degrees centigrade so that hydrogen atoms fuse together, releasing energy. Fission reactors work by splitting atoms at much lower temperatures.
The advantage of fusion reactors is that they create almost no radioactive waste and high-level radioactive material to potentially leak into the environment. That means disasters like Chernobyl or Fukushima are impossible because plasma simply fizzles out if it escapes.
Fusion energy would also not produce weapons-grade products that proliferate nuclear arms. It is fueled by deuterium (“heavy water”), which is extracted from seawater, and tritium, which is created within the reactor, so there is no problem with security of supply either.
A test fusion reactor based a tokamak design, the International Thermonuclear Experimental Reactor (ITER), is about 10 years away from operation in the South of France. Its aim is to prove the scientific and technological feasibility of fusion energy. MIT also plans to create new lower-cost, compact version of a tokamak fusion reactor, also based on improved superconductors, which are required to produce the high current needed to produce magnetic fields.
“Fission, fusion, or fossil fuels are the only practical options for reliable large-scale base-load energy sources,” said Professor Damian Hampshire, of the Centre for Material Physics at Durham University, who led the study. “Calculating the cost of a fusion reactor is complex, given the variations in the cost of raw materials and exchange rates. However, this work is a big step in the right direction” he said.
The potential for reducing the Cost of Electricity (CoE) by using High Temperature Superconductors (HTS) in the Toroidal Field (TF) coils of a fusion tokamak power plant has been investigated using a new HTS module in the PROCESS systems code. We report the CoE and the design of HTS tokamaks that have been optimised by minimising the major radius of the plasma. Potential future improvements in both the superconducting properties and the structural materials for TF coils operating at 4.8 K and 30 K are considered. Increasing the critical current density by a factor of 10 (with a commensurate reduction in costs kA−1 m−1) results in a CoE 4.4% less than equivalent tokamaks using current low temperature superconductors (LTS). If the yield strength of the TF casing material is increased by 40% to 1400 MPa, the CoE is further reduced by 3.4%. Implementing both improvements and operating the TF coils at 4.8 K leads to CoE of 19.1 (10.1) €cent kW−1 h−1 for a 500 MW (1.5 GW) HTS reactor compared to 20.7 (11.1) €cent kW−1 h−1 for an LTS reactor (2013 costs). Operating the HTS TF coils at 30 K with both improvements, gives a similar CoE for HTS and LTS tokamaks.