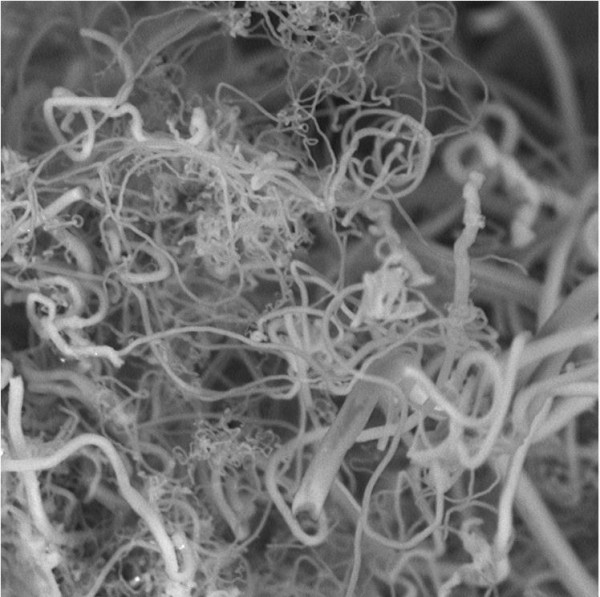
Researchers are removing a greenhouse gas from the air while generating carbon nanofibers like these (credit: Stuart Licht, Ph.D)
A research team of chemists at George Washington University has developed a technology that can economically convert atmospheric CO2 directly from the air into highly valued carbon nanofibers for industrial and consumer products — converting an anthropogenic greenhouse gas from a climate change problem to a valuable commodity, they say.
The team presented their research today (Aug. 19) at the 250th National Meeting & Exposition of the American Chemical Society (ACS).
“Such nanofibers are used to make strong carbon composites, such as those used in the Boeing Dreamliner, as well as in high-end sports equipment, wind turbine blades and a host of other products,” said Stuart Licht, Ph.D., team leader.
Previously, the researchers had made fertilizer and cement without emitting CO2, which they reported. Now, the team, which includes postdoctoral fellow Jiawen Ren, Ph.D., and graduate student Jessica Stuart, says their research could shift CO2 from a global-warming problem to a feed stock for the manufacture of in-demand carbon nanofibers.
Licht calls his approach “diamonds from the sky.” That refers to carbon being the material that diamonds are made of, and also hints at the high value of the products, such as carbon nanofibers.
A low-energy, high-efficiency process
The researchers claim this low-energy process can be run efficiently, using only a few volts of electricity, sunlight, and a whole lot of carbon dioxide. The system uses electrolytic syntheses to make the nanofibers. Here’s how:
- To power the syntheses, heat and electricity are produced through a hybrid and extremely efficient concentrating solar-energy system. The system focuses the sun’s rays on a photovoltaic solar cell to generate electricity and on a second system to generate heat and thermal energy, which raises the temperature of an electrolytic cell.
- CO2 is broken down in a high-temperature electrolytic bath of molten carbonates at 1,380 degrees F (750 degrees C).
- Atmospheric air is added to an electrolytic cell.
- The CO2 dissolves when subjected to the heat and direct current through electrodes of nickel and steel.
- The carbon nanofibers build up on the steel electrode, where they can be removed.
Licht estimates electrical energy costs of this “solar thermal electrochemical process” to be around $1,000 per ton of carbon nanofiber product. That means the cost of running the system is hundreds of times less than the value of product output, he says.
Decreasing CO2 to pre-industrial-revolution levels
“We calculate that with a physical area less than 10 percent the size of the Sahara Desert, our process could remove enough CO2 to decrease atmospheric levels to those of the pre-industrial revolution within 10 years,” he says.
At this time, the system is experimental. Licht’s biggest challenge will be to ramp up the process and gain experience to make consistently sized nanofibers. “We are scaling up quickly,” he adds, “and soon should be in range of making tens of grams of nanofibers an hour.”
Licht explains that one advance the group has recently achieved is the ability to synthesize carbon fibers using even less energy than when the process was initially developed. “Carbon nanofiber growth can occur at less than 1 volt at 750 degrees C, which for example is much less than the 3–5 volts used in the 1,000 degree C industrial formation of aluminum,” he says.
No published details on overall energy costs and efficiency are yet available (to be updated).
Abstract of New approach to carbon dioxide utilization: The carbon molten air battery
As the levels of carbon dioxide (CO2) increase in the Earth’s atmosphere, the effects on climate change become increasingly apparent. As the demand to reduce our dependence on fossils fuels and lower our carbon emissions increases, a transition to renewable energy sources is necessary. Cost effective large-scale electrical energy storage must be established for renewable energy to become a sustainable option for the future. We’ve previously shown that carbon dioxide can be captured directly from the air at solar efficiencies as high as 50%, and that carbon dioxide associated with cement formation and the production of other commodities can be electrochemically avoided in the STEP process.1-3
The carbon molten air battery, presented by our group in late 2013, is attractive due to its scalability, location flexibility, and construction from readily available resources, providing a battery that can be useful for large scale applications, such as the storage of renewable electricity.4
Uncommonly, the carbon molten air battery can utilize carbon dioxide directly from the air:
(1) charging: CO2(g) -> C(solid) + O2(g)
(2) discharging: C(solid) + O2(g) -> CO2(g)
More specifically, in a molten carbonate electrolyte containing added oxide, such as lithium carbonate with lithium oxide, the 4 electron charging reaction eq. 1 approaches 100% faradic efficiency and can be described as the following two equations:
(1a) O2-(dissolved) + CO2(g) -> CO32-(molten)
(1b) CO32-(molten) -> C(solid) + O2(g) + O2-(dissolved)
Thus, powered by carbon formed directly from the CO2 in our earth’s atmosphere, the carbon molten air battery is a viable system to provide large-scale energy storage.
1S. Licht, ”Efficient Solar-Driven Synthesis, Carbon Capture, and Desalinization, STEP: Solar Thermal Electrochemical Production of Fuels, Metals, Bleach,” Advanced Materials, 47, 5592 (2011).
2S. Licht, H. Wu, C. Hettige, B. Wang, J. Lau, J. Asercion, J. Stuart “STEP Cement: Solar Thermal Electrochemical Production of CaO without CO2 emission,” Chemical Communications, 48, 6019 (2012).
3S. Licht, B. Cui, B. Wang, F.-F. Li, J. Lau, S. Liu,” Ammonia synthesis by N2 and steam electrolysis in molten hydroxide suspensions of nanoscale Fe2O3,” Science, 345, 637 (2014).
4S. Licht, B. Cui, J. Stuart, B. Wang, J. Lau, “Molten Air Batteries – A new, highest energy class of rechargeable batteries,” Energy & Environmental Science, 6, 3646 (2013).