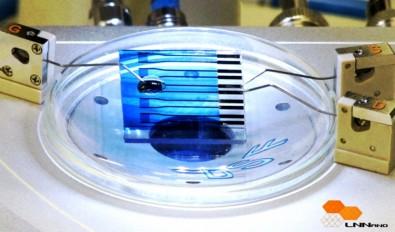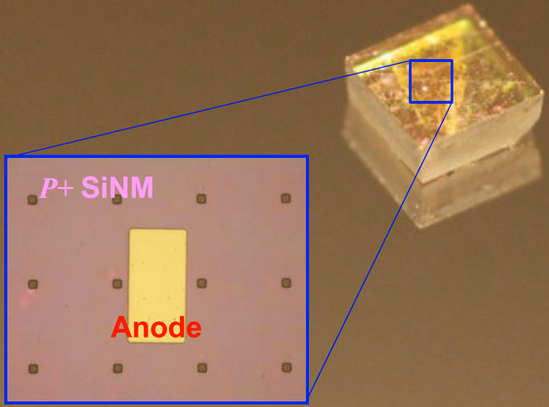
A diode array bonded to a natural single crystalline diamond plate. Inset: deposited anode metal on top of doped silicon nanomembrane. (credit: Jung-Hun Seo)
Researchers have developed a new method for doping (integrating elements to change a semiconductor’s properties) single crystals of diamond with boron at relatively low temperatures, without degradation.
Diamonds have properties that could make them ideal semiconductors for power electronics. They can handle high voltages and power, and electrical currents also flow through diamonds quickly, meaning the material would make for energy-efficient devices. And they are thermally conductive, which means diamond-based devices would dissipate heat quickly and easily (no need for bulky, expensive cooling methods). However. diamond’s rigid crystalline structure makes doping difficult.*
Doping a diamond with boron
Zhengqiang (Jack) Ma, a University of Wisconsin-Madison electrical and computer engineering professor, and his colleagues describe a solution in the Journal of Applied Physics, from AIP Publishing.
They discovered that if you bond a single-crystal diamond with a piece of silicon doped with boron, and heat it to 800 degrees Celsius (low compared to conventional techniques), the boron atoms will migrate from the silicon to the diamond. It turns out that the boron-doped silicon has defects such as vacancies, where an atom is missing in the lattice structure. Carbon atoms from the diamond will fill those vacancies, leaving empty spots for boron atoms.
This technique also allows for selective doping, which means more control when making devices. You can choose where to dope a single-crystal diamond simply by bonding the silicon to that spot.
The new method currently only works for P-type doping, where the semiconductor is doped with an element that provides positive charge carriers (in this case, the absence of electrons, called holes). The researchers are already working on a simple device using P-type single-crystal diamond semiconductors.
But to make electronic devices like transistors, you need N-type doping, which gives the semiconductor negative charge carriers (electrons). And other barriers remain: diamond is expensive and single crystals are very small.
Still, Ma says, achieving P-type doping is an important step, and might inspire others to find solutions for the remaining challenges. Eventually, he said, single-crystal diamond could be useful everywhere — perfect, for instance, for controlling power in the electrical grid.
* Currently, you can dope diamond by coating the crystal with boron and heating it to 1450 degrees Celsius. But it’s difficult to remove the boron coating at the end. This method only works on diamonds consisting of multiple crystals stuck together. Because such polydiamonds have irregularities between the crystals, single crystals would be superior semiconductors. You can dope single crystals by injecting boron atoms while growing the crystals artificially. The problem is the process requires powerful microwaves that can degrade the quality of the crystal.
Abstract of Thermal diffusion boron doping of single-crystal natural diamond
With the best overall electronic and thermal properties, single crystal diamond (SCD) is the extreme wide bandgap material that is expected to revolutionize power electronics and radio-frequency electronics in the future. However, turning SCD into useful semiconductors requires overcoming doping challenges, as conventional substitutional doping techniques, such as thermal diffusion and ion implantation, are not easily applicable to SCD. Here we report a simple and easily accessible doping strategy demonstrating that electrically activated, substitutional doping in SCD without inducing graphitization transition or lattice damage can be readily realized with thermal diffusion at relatively low temperatures by using heavily dopedSi nanomembranes as a unique dopant carrying medium. Atomistic simulations elucidate a vacancyexchange boron doping mechanism that occurs at the bonded interface between Si and diamond. We further demonstrate selectively doped high voltage diodes and half-wave rectifier circuits using such dopedSCD. Our new doping strategy has established a reachable path toward using SCDs for future high voltage power conversion systems and for other novel diamond based electronic devices. The novel dopingmechanism may find its critical use in other wide bandgap semiconductors.