Rice University postdoctoral researcher Pei Dong holds a sample of SAC, a new form of self-adapting composite. The material has the ability to heal itself and to regain its original shape after extraordinary compression. (credit: Jeff Fitlow/Rice University)
A flexible adaptive material invented at Rice University combines self-healing and reversible self-stiffening properties.
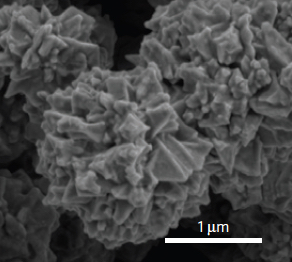
The material, called SAC (for self-adaptive composite), consists of sticky, micron-scale rubber balls that form a solid matrix. The researchers made SAC by mixing two polymers and a solvent that evaporates when heated, leaving a porous mass of gooey spheres. When cracked, the matrix quickly heals, over and over. And like a sponge, it returns to its original form after compression.
SAC may be a useful biocompatible material for tissue engineering or a lightweight, defect-tolerant structural component.
The labs of Rice materials scientists Pulickel Ajayan and Jun Lou led the study that appears in the American Chemical Society journal ACS Applied Materials and Interfaces. They suggested
Other “self-healing” materials encapsulate liquid in solid shells that leak their healing contents when cracked.
In SAC, tiny spheres of polyvinylidene fluoride (PVDF) encapsulate much of the liquid. The viscous polydimethylsiloxane (PDMS) further coats the entire surface. The spheres are extremely resilient, Lou said, as their thin shells deform easily. Their liquid contents enhance their viscoelasticity, a measure of their ability to absorb the strain and return to their original state, while the coatings keep the spheres together. The spheres also have the freedom to slide past each other when compressed, but remain attached.

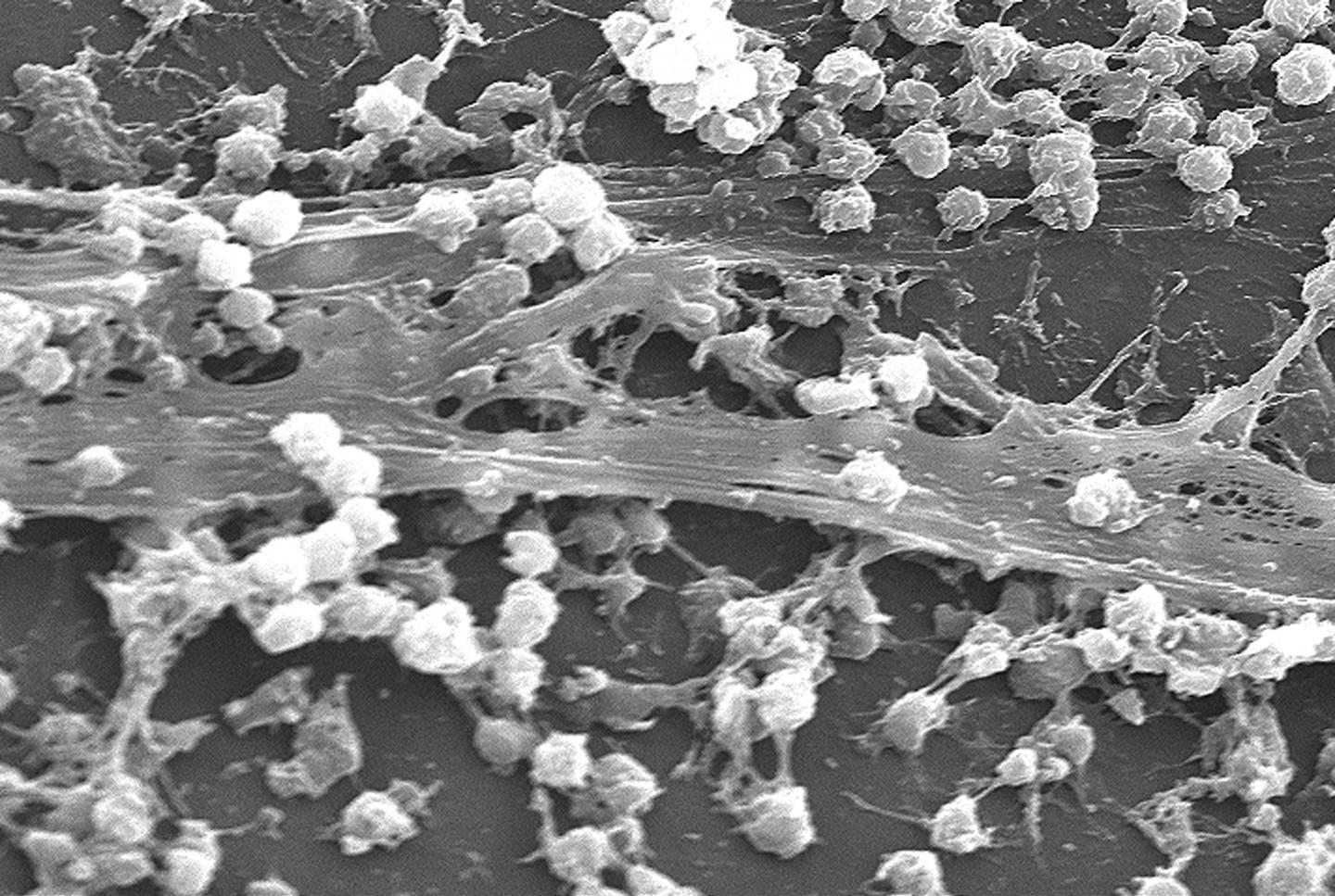
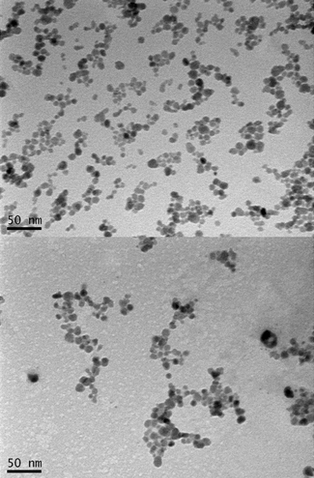
Characterization of the hierarchical solid-liquid composite. (a) Image of solid-liquid SAC. (b) SEM image of the porous SAC. (c) High magnification SEM image of the shell of the bead. (credit: Pei Dong et al./ACS Appl. Mater. Interfaces)
“The sample doesn’t give you the impression that it contains any liquid,” he said. “That’s very different from a gel. This is not really squishy; it’s more like a sugar cube that you can compress quite a lot. The nice thing is that it recovers.”
Making SAC is simple, and the process can be tuned — a little more liquid or a little more solid — to regulate the product’s mechanical behavior. The polymer components begin as powder and viscous liquid. With the addition of a solvent and controlled heating, the PDMS stabilizes into solid spheres that provide the reconfigurable internal structure.
In tests, Rice scientists found a maximum of 683 percent increase in the material’s storage modulus — a size-independent parameter used to characterize self-stiffening behavior. This is much larger than that reported for solid composites and other materials, they said.
Rice University | Self-adaptive material heals itself, stays tough
Abstract of A Solid-liquid Self-adaptive Polymeric Composite
A solid-liquid self-adaptive composite (SAC) is synthesized using a simple mixing-evaporation protocol, with Poly(dimethylsiloxane) (PDMS) and Poly(vinylidene fluoride) (PVDF) as active constituents. SAC exists as a porous solid containing a near equivalent distribution of the solid (PVDF)-liquid (PDMS) phases, with the liquid encapsulated and stabilized within a continuous solid network percolating throughout the structure. The pores, liquid and solid phases form a complex hierarchical structure, which offers both mechanical robustness and a significant structural adaptability under external forces. SAC exhibits attractive self-healing properties during tension, and demonstrates reversible self-stiffening properties under compression with a maximum of seven-fold increase seen in the storage modulus. Compared to existing self-healing and self-stiffening materials, SAC offers distinct advantages in the ease of fabrication, high achievable storage modulus, and reversibility. Such materials could provide a new class of adaptive materials system with multi-functionality, tunability, and scale-up potentials.