MIT | Microscope creates near-real-time videos of nanoscale processes
MIT engineers have designed an atomic force microscope (AFM) that scans images 2,000 times faster than existing commercial models. Operating at near-real-time-video speed, it can capture structures as small as a fraction of a nanometer from single strands of DNA down to individual hydrogen bonds.
Existing AFMs have similar spatial resolution but function at slow speeds.
In one dramatic demonstration of the instrument’s capabilities (see video), the researchers scanned a 70- by-70-micrometers sample of calcite as it was first immersed in deionized water and later exposed to sulfuric acid. Over a period of several seconds, the team observed the acid eating away at the calcite, expanding existing nanometer-sized pits in the material that quickly merged and led to a layer-by-layer removal of calcite along the material’s crystal pattern.
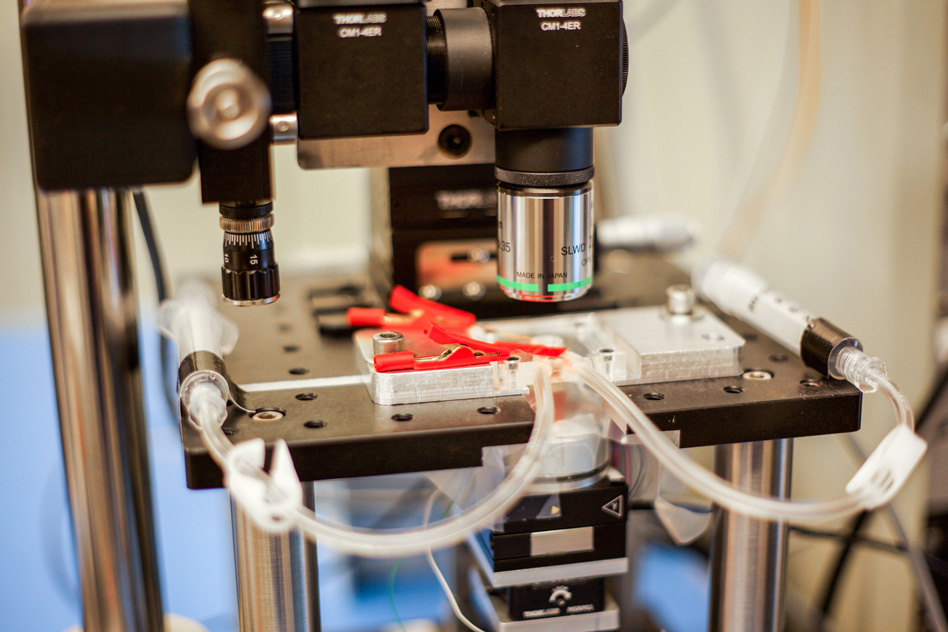
The new MIT high-speed microscope produces images of chemical processes taking place at the nanoscale at a rate that is close to real-time video. This closeup shot of the microscope shows transparent tubes used to inject various liquids into the imaging environment. This liquid can be water, acid, buffer solution for live bacteria, cells, or electrolytes in an electrochemical process. Researchers use one as an inlet and the other as an outlet to circulate and refresh the solutions throughout the experiment. (credit: Jose-Luis Olivares/MIT)
Kamal Youcef-Toumi, a professor of mechanical engineering at MIT, says the instrument’s sensitivity and speed will enable scientists to watch atomic-sized processes play out as high-resolution “movies” for the first time.
“People can see, for example, condensation, nucleation, dissolution, or deposition of material, and how these happen in real-time — things that people have never seen before,” Youcef-Toumi says. “This is fantastic to see these details emerging. And it will open great opportunities to explore all of this world that is at the nanoscale.”
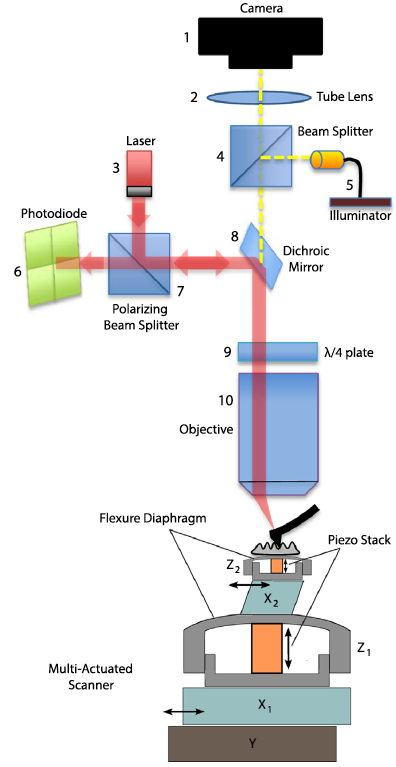
A schematic of the AFM (credit: I. Soltani Bozchalooi et al./Ultramicroscopy)
The group’s design and images, which are based on the PhD work of Iman Soltani Bozchalooi, now a postdoc in the Department of Mechanical Engineering, are published in the journal Ultramicroscopy.
Atomic force microscopes typically scan samples using an ultrafine probe, or needle, that skims along the surface of a sample, tracing its topography, similarly to how a blind person reads Braille. Samples sit on a movable platform, or scanner, that moves the sample laterally and vertically beneath the probe.
Because AFMs scan incredibly small structures, the instruments have to work slowly, line by line, to avoid any sudden movements that could alter the sample or blur the image. Such conventional microscopes typically scan only about one to two lines per second.
To speed up the scanning process, scientists have built platforms that scan samples more quickly, but over a smaller area, and the platforms don’t allow scientists to zoom out to see a wider view or study larger features.
Synchronized Scanners
The main innovation of the new design is a multi-actuated scanner. The sample platform incorporates both a smaller, speedier scanner and a larger, slower scanner for every direction, which work together as one system to scan a wide 3-D region at high speed.
The microscope operates at about eight to 10 frames per second and can scan across hundreds of microns and image features that are several microns high.
“We want to go to real video, which is at least 30 frames per second,” Youcef-Toumi says. “Hopefully we can work on improving the instrument and controls so that we can do video-rate imaging while maintaining its large range and keeping it user-friendly.”
Abstract of Design and control of multi-actuated atomic force microscope for large-range and high-speed imaging
This paper presents the design and control of a high-speed and large-range atomic force microscopy (AFM). A multi-actuation scheme is proposed where several nano-positioners cooperate to achieve the range and speed requirements. A simple data-based control design methodology is presented to effectively operate the AFM scanner components. The proposed controllers compensate for the coupled dynamics and divide the positioning responsibilities between the scanner components. As a result, the multi-actuated scanner behavior is equivalent to that of a single X–Y–Z positioner with large range and high speed. The scanner of the designed AFM is composed of five nano-positioners, features 6 μm out-of-plane and 120 μm lateral ranges and is capable of high-speed operation. The presented AFM has a modular design with laser spot size of 3.5 μm suitable for small cantilever, an optical view of the sample and probe, a conveniently large waterproof sample stage and a 20 MHz data throughput for high resolution image acquisition at high imaging speeds. This AFM is used to visualize etching of calcite in a solution of sulfuric acid. Layer-by-layer dissolution and pit formation along the crystalline lines in a low pH environment is observed in real time.
Abstract of Multi-actuation and PI control: A simple recipe for high-speed and large-range atomic force microscopy
High speed atomic force microscopy enables observation of dynamic nano-scale processes. However, maintaining a minimal interaction force between the sample and the probe is challenging at high speed specially when using conventional piezo-tubes. While rigid AFM scanners are operational at high speeds with the drawback of reduced tracking range, multi-actuation schemes have shown potential for high-speed and large-range imaging. Here we present a method to seamlessly incorporate additional actuators into conventional AFMs. The equivalent behavior of the resulting multi-actuated setup resembles that of a single high-speed and large-range actuator with maximally flat frequency response. To achieve this, the dynamics of the individual actuators and their couplings are treated through a simple control scheme. Upon the implementation of the proposed technique, commonly used PI controllers are able to meet the requirements of high-speed imaging. This forms an ideal platform for retroactive enhancement of existing AFMs with minimal cost and without compromise on the tracking range. A conventional AFM with tube scanner is retroactively enhanced through the proposed method and shows an order of magnitude improvement in closed loop bandwidth performance while maintaining large range. The effectiveness of the method is demonstrated on various types of samples imaged in contact and tapping modes, in air and in liquid.