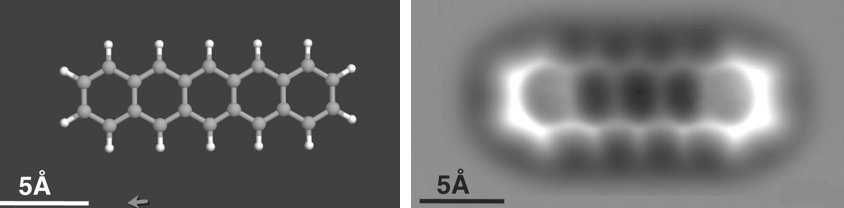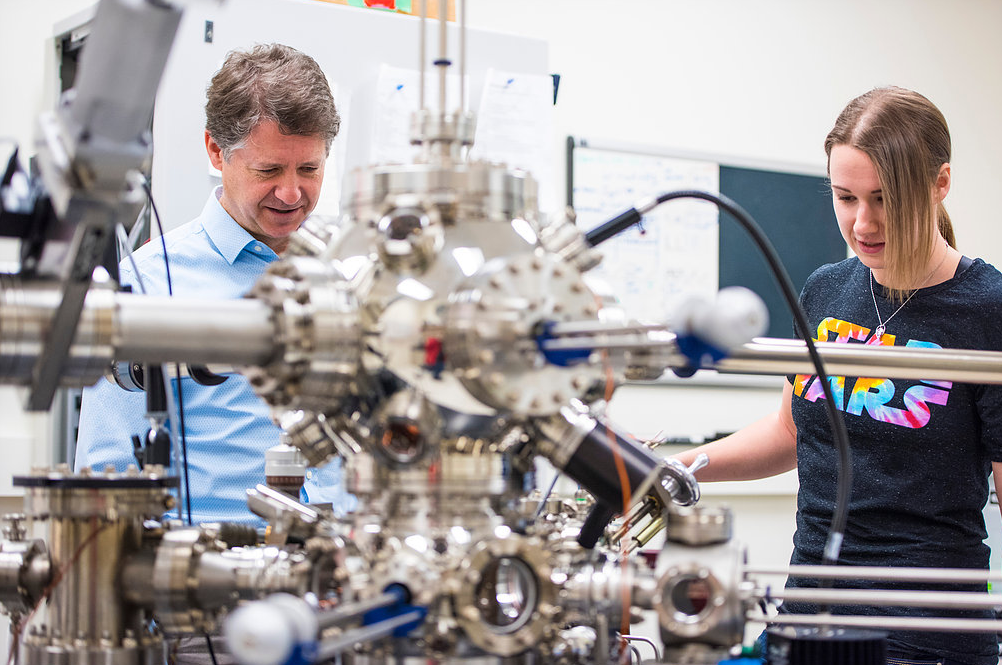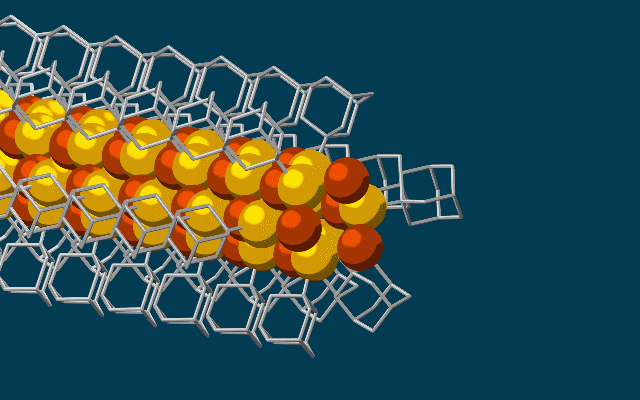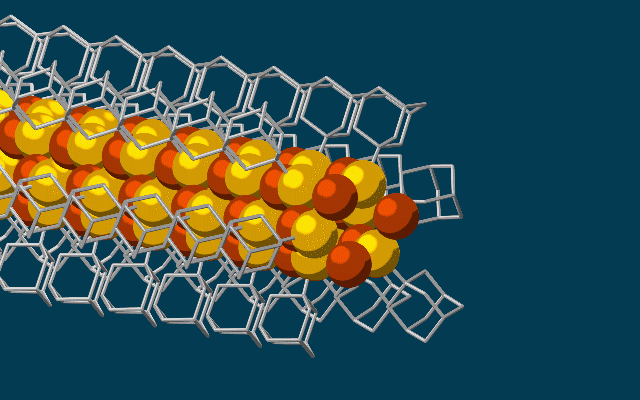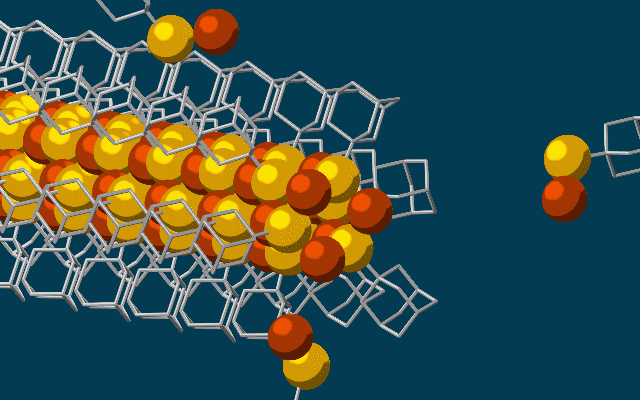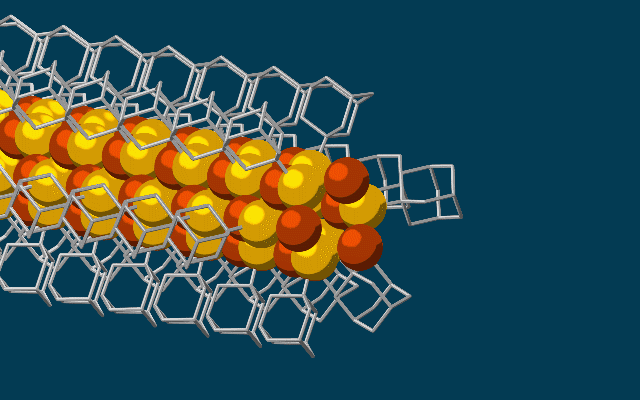
Scanning tunneling microscope image of a single atom of holmium, an element that researchers used as a magnet to store one bit of data. (credit: IBM Research — Almaden)
An international team led by IBM has created the world’s smallest magnet, using a single atom of rare-earth element holmium, and stored one bit of data on it over several hours.
The achievement represents the ultimate limit of the classical approach to high-density magnetic storage media, according to a paper published March 8 in the journal Nature.
Currently, hard disk drives use about 100,000 atoms to store a single bit. The ability to read and write one bit on one atom may lead to significantly smaller and denser storage devices in the future. (The researchers are currently working in an ultrahigh vacuum at 1.2 K (a temperature near absolute zero.)
Using a scanning tunneling microscope* (STM), the researchers also showed that a device using two magnetic atoms could be written and read independently, even when they were separated by just one nanometer.
IBM microscope mechanic Bruce Melior at scanning tunneling microscope, used to view and manipulate atoms (credit: IBM Research — Almaden)
The researchers believe this tight spacing could eventually yield magnetic storage that is 1,000 times denser than today’s hard disk drives and solid state memory chips. So they could one day store 1,000 times more information in the same space. That means data centers, computers, and personal devices would be radically smaller and more powerful.
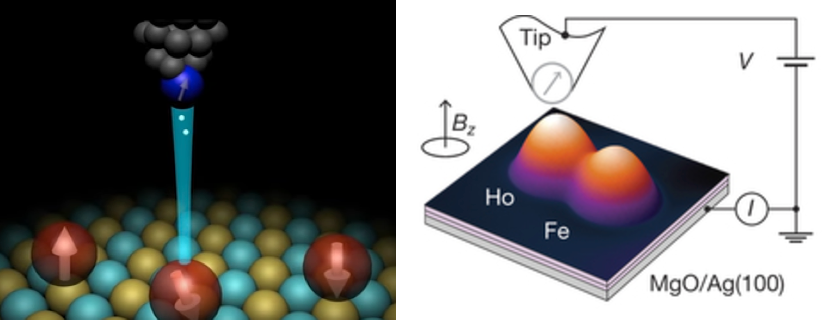
Single-atom write and read operations. (Left) To write the data onto the holmium atom, a pulse of electric current from the magnetized tip of a scanning tunneling microscope (STM) is used to flip the orientation of the atom’s field between a 0 or 1. The STM is also used to read it. (Right) A second read-out method used an iron atom as a magnetic sensor, which also allowed the team to read out multiple bits at the same time, making it more practical than an STM. (credit: IBM Research and Fabian D. Natterer et al./Nature)
Researchers at EPFL in Switzerland, University of Chinese Academy of Sciences in Hong Kong, University of Göttingen in Germany, Universität Zürich in Switzerland, Institute of Basic Science, Center for Quantum Nanoscience in South Korea, and Ewha Womans University in South Korea were also on the research team.
* The STM was developed in 1981, earning its inventors, Gerd Binnig and Heinrich Rohrer (at IBM Zürich), the Nobel Prize in Physics in 1986. IBM is planning future scanning tunneling microscope studies to investigate the potential of performing quantum information processing using individual magnetic atoms. Earlier this week, IBM announced it will be building the world’s first commercial quantum computers for business and science.
IBM Research | IBM Research Created the World’s Smallest Magnet — an Atom