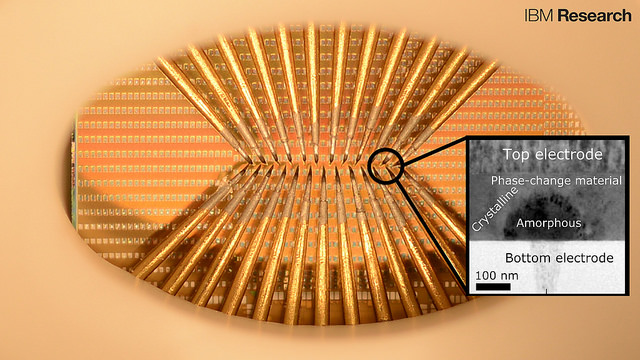
Liquid metal material with metallic core and semiconducting skin (credit: RMIT University)
Imagine a soft liquid-metal material right out of the T-1000 Terminator movie character. One that can morph itself into different self-propelling soft electronic circuits that act like live cells, communicating with each other.
Using a liquid metallic core* and semiconducting skin, such a soft material might be used to make instant flexible 3D electronic displays. Or morph into self-propelled biomedical diagnostic sensors, for example, reconfiguring themselves on demand, say RMIT University researchers.
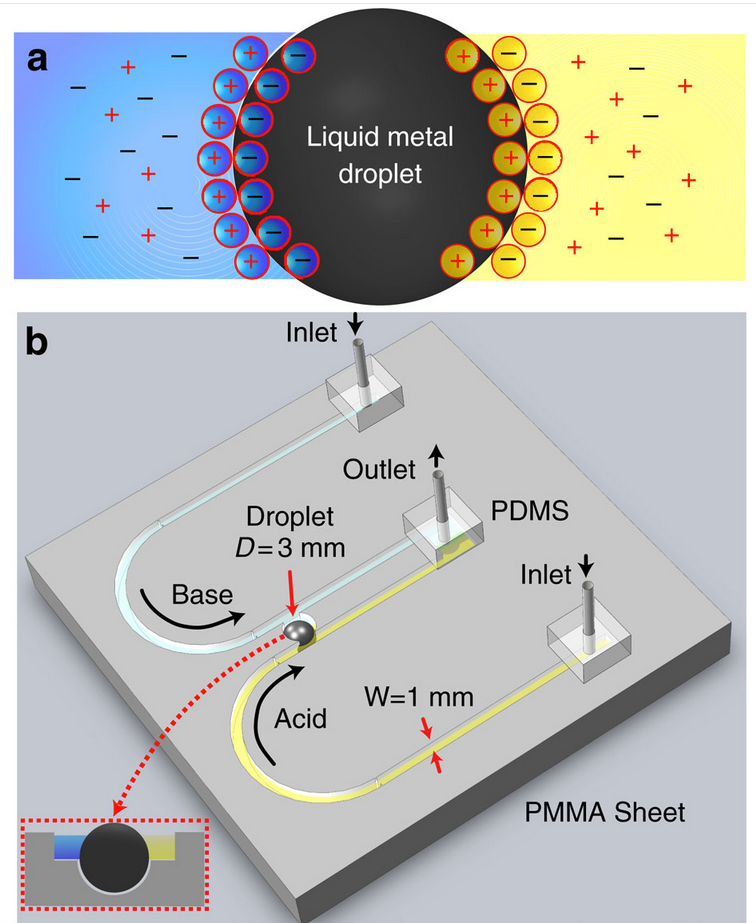
Diagram of ionic-imbalance-induced self-propulsion of liquid metals. (a) Schematic of the droplet and arrangement of ions, forming the EDL. (b) Schematic of the experimental setup showing two U-shaped open-top (see inset) inlet channels, which extend in parallel and join at an outlet. Two channels carry different types of electrolytes (acidic in yellow and basic in blue). Two parallel flows come in contact with the Galinstan droplet (3 mm diameter) residing in a recess. (credit: Ali Zavabeti et al./Nature Communications)
Morphing metal — more like live cells
To achieve that magic, Professor Kourosh Kalantar-zadeh and his group first immersed liquid-metal droplets in water. The droplets were able to move about freely in three dimensions, driven by pH (acid or base) or ionic (electric charge) concentration gradients across a liquid metal droplet, which induced deformation and surface flow.
“We adjusted the concentrations of acid, base, and salt components in the water and investigated the effect. Simply tweaking the water’s chemistry made the liquid metal droplets move and change shape, without any need for external mechanical, electronic, or optical stimulants.”
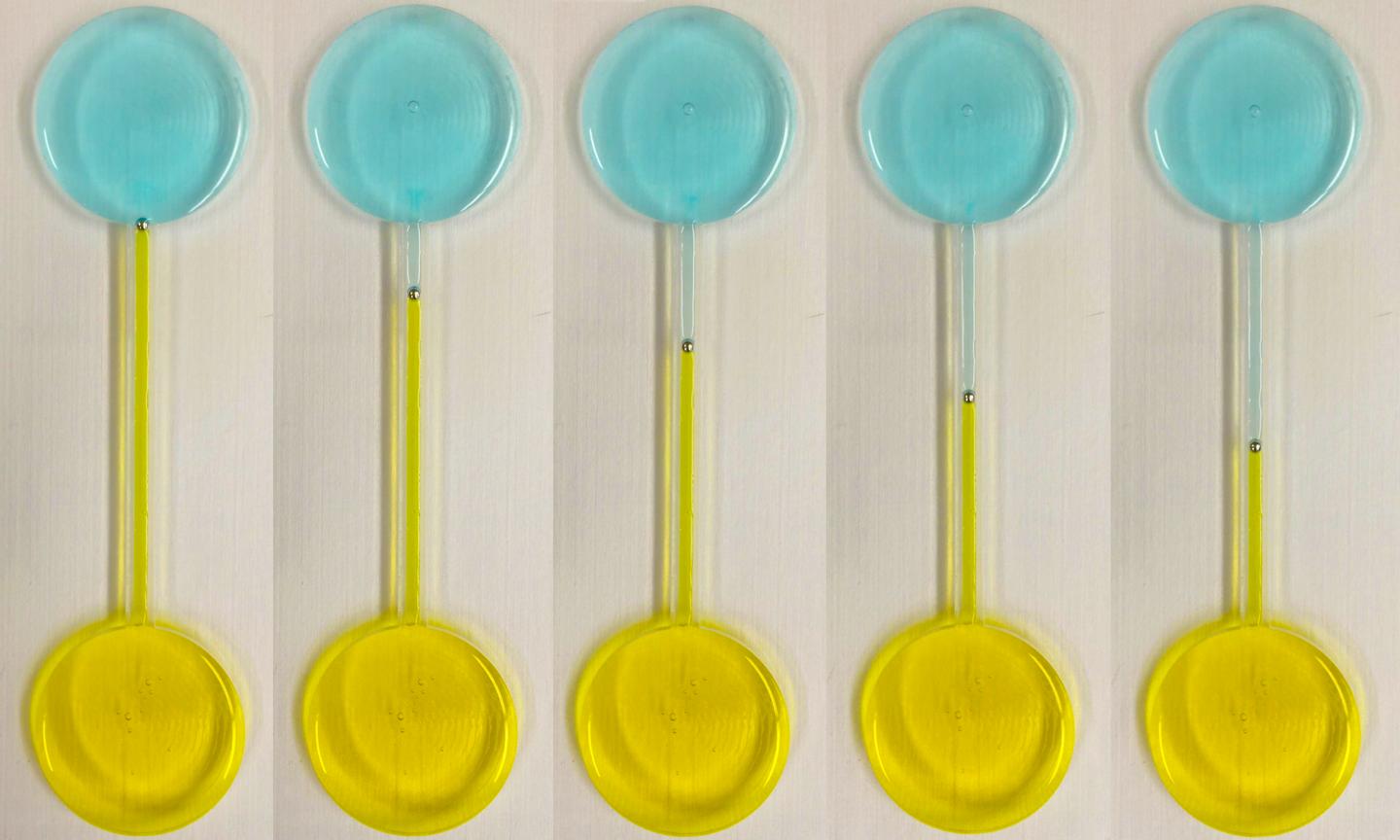
No, these are not lollipops. These devices demonstrate continuous motion of a self-propelling liquid metal droplet under a pH gradient, shown at different time intervals (left to right). The droplet is placed in a fluidic channel, midway between two reservoirs filled with different acid and base electrolytes. (credit: RMIT University)
The elastic electronic soft circuit systems act more like live cells, moving around autonomously and communicating with each other to form new circuits, rather than being stuck in one configuration.
“Using this discovery, we were able to create moving objects, switches, and pumps that could operate autonomously — self-propelling liquid metals driven by the composition of the surrounding fluid,” Kalantar-zadeh said. “Eventually, using the fundamentals of this discovery, it may be possible to build a 3D liquid metal humanoid on demand.”
The research was published August 4 in open-access Nature Communications.
* Galinstan, an alloy of of 68.5% gallium, 21.5% indium, and 10% tin, is used as the model liquid metal. Galinstan’s melting point can be lowered to below 0 °C (32 °F).
RMIT University | Liquid metals propel future electronics | RMIT University
Abstract of Ionic imbalance induced self-propulsion of liquid metals
Components with self-propelling abilities are important building blocks of small autonomous systems and the characteristics of liquid metals are capable of fulfilling self-propulsion criteria. To date, there has been no exploration regarding the effect of electrolyte ionic content surrounding a liquid metal for symmetry breaking that generates motion. Here we show the controlled actuation of liquid metal droplets using only the ionic properties of the aqueous electrolyte. We demonstrate that pH or ionic concentration gradients across a liquid metal droplet induce both deformation and surface Marangoni flow. We show that the Lippmann dominated deformation results in maximum velocity for the self-propulsion of liquid metal droplets and illustrate several key applications, which take advantage of such electrolyte-induced motion. With this finding, it is possible to conceive the propulsion of small entities that are constructed and controlled entirely with fluids, progressing towards more advanced soft systems.